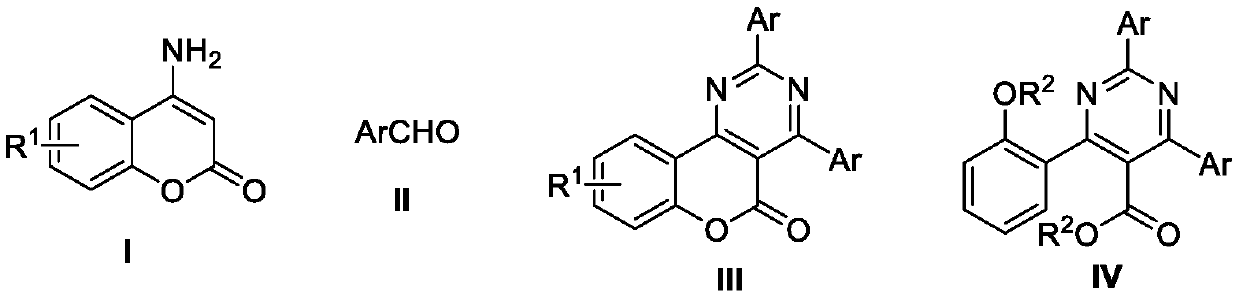
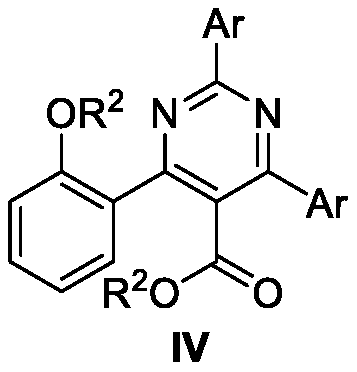
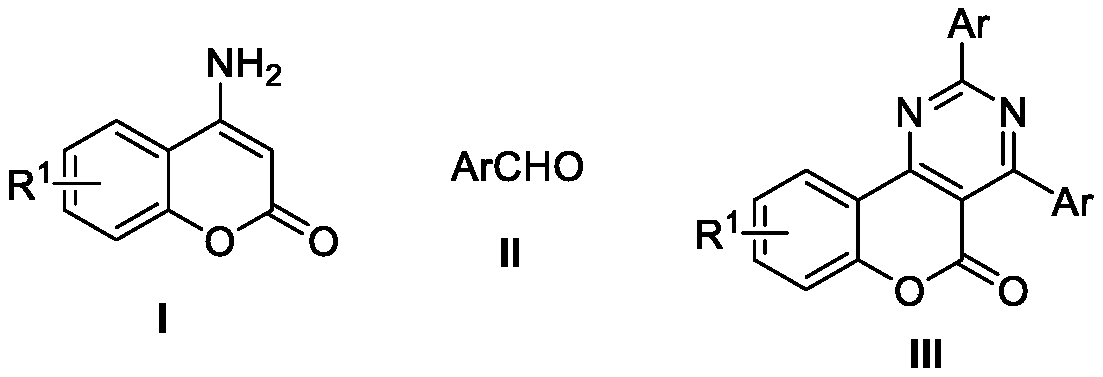
Method for synthesizing all-carbon-substituted pyrimidine derivative
A technology for compounds and products, applied in the field of synthesizing all-carbon substituted pyrimidine derivatives, can solve the problems of high functionalization of raw materials and difficulty in obtaining
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The synthesis of 2,6-bis(2-tolyl)-4-(2-ethoxyphenyl)pyrimidine-5-carboxylic acid ethyl ester (the structural formula of the compound is shown in formula IV-1), including the following steps:
[0024] Into the reaction tube, add 4-aminocoumarin (0.2 mmol), ammonium iodide (0.24 mmol), and solid benzaldehyde (0.6 mmol) successively, and use a double-row tube system to remove oxygen / refill argon and circulate three times. Dimethyl sulfoxide (0.2mmol, 18μL) and chlorobenzene (0.5mL) were sequentially injected through a syringe, and the tube was sealed and reacted at 150°C for 24h. The intermediate obtained from the reaction was cooled to room temperature, solid sodium hydroxide (0.48 mmol, 20 mg) and dimethyl sulfoxide (0.6 mL) were added, and stirred at room temperature (20-30°C) for 0.5 hours, and then bromoethane ( 0.48mmol, 52mg), and continue to stir for 3 hours. Treated with ethyl acetate and water, and separated by thin layer chromatography (petroleum ether / ethyl aceta...
Embodiment 2
[0028] In the synthesis of the compound represented by formula IV-2, 4-tolualdehyde replaces the benzaldehyde in Example 1, and other reaction conditions are the same as in Example 1, and the yield is 80%;
[0029]
[0030] White solid, m.p.140-142℃. 1 H NMR (400 MHz, CDCl 3 )δ8.49(d,J=8.2 Hz,2H), 7.65(d,J=8.1 Hz,2H), 7.54(dd,J=7.5,1.6 Hz,1H),7.40(td,J=8.3,1.7 Hz, 1H), 7.29–7.26 (m, 4H), 7.09 (t, J = 7.5 Hz, 1H), 6.93 (d, J = 8.3 Hz, 1H), 4.02–3.94 (m, 4H), 2.42 (s ,3H),2.41(s,3H),1.25(t,J=7.0 Hz,3H),0.87(t,J=7.1 Hz,3H). 13 C NMR(100MHz, CDCl 3 )δ167.7, 164.4, 164.2, 163.9, 156.0, 141.3, 139.8, 135.9, 134.7, 130.7, 129.2(2C), 129.0(2C), 128.8(2C), 128.7(2C), 128.2, 123.5, 120.7, 111.8, 64.1 ,61.1,21.6,21.4,14.5,13.4.
Embodiment 3
[0032] In the synthesis of the compound represented by formula IV-3, 4-fluorobenzaldehyde replaces the benzaldehyde in Example 1, and other reaction conditions are the same as in Example 1, and the yield is 82%;
[0033]
[0034] White solid, m.p.114-115℃. 1 H NMR (400 MHz, CDCl 3 )δ8.60(dd,J=8.4,5.9 Hz,2H),7.74(dd,J=8.5,5.4 Hz,2H),7.54(d,J=7.4 Hz,1H),7.42(t,J=7.8 Hz,1H),7.19–7.08(m,5H),6.94(d,J=8.3 Hz,1H),4.03–3.95(m,4H),1.26(t,J=7.0 Hz, 3H),0.89(t ,J=7.1 Hz,3H). 13 C NMR(101 MHz, CDCl 3 )δ167.4, 164.9 (J = 249.5 Hz), 163.9 (J = 258.5 Hz), 164.7, 163.4, 163.0, 155.9, 134.6 (J = 3.3 Hz), 133.3 (J = 2.8 Hz), 131.03, 130.9, 130.8, 130.7 ,130.72,127.76,123.71,120.76,115.5 (J = 21.5 Hz), 115.4 (J = 21.7 Hz), 111.7, 64.1, 61.3, 14.4, 13.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com