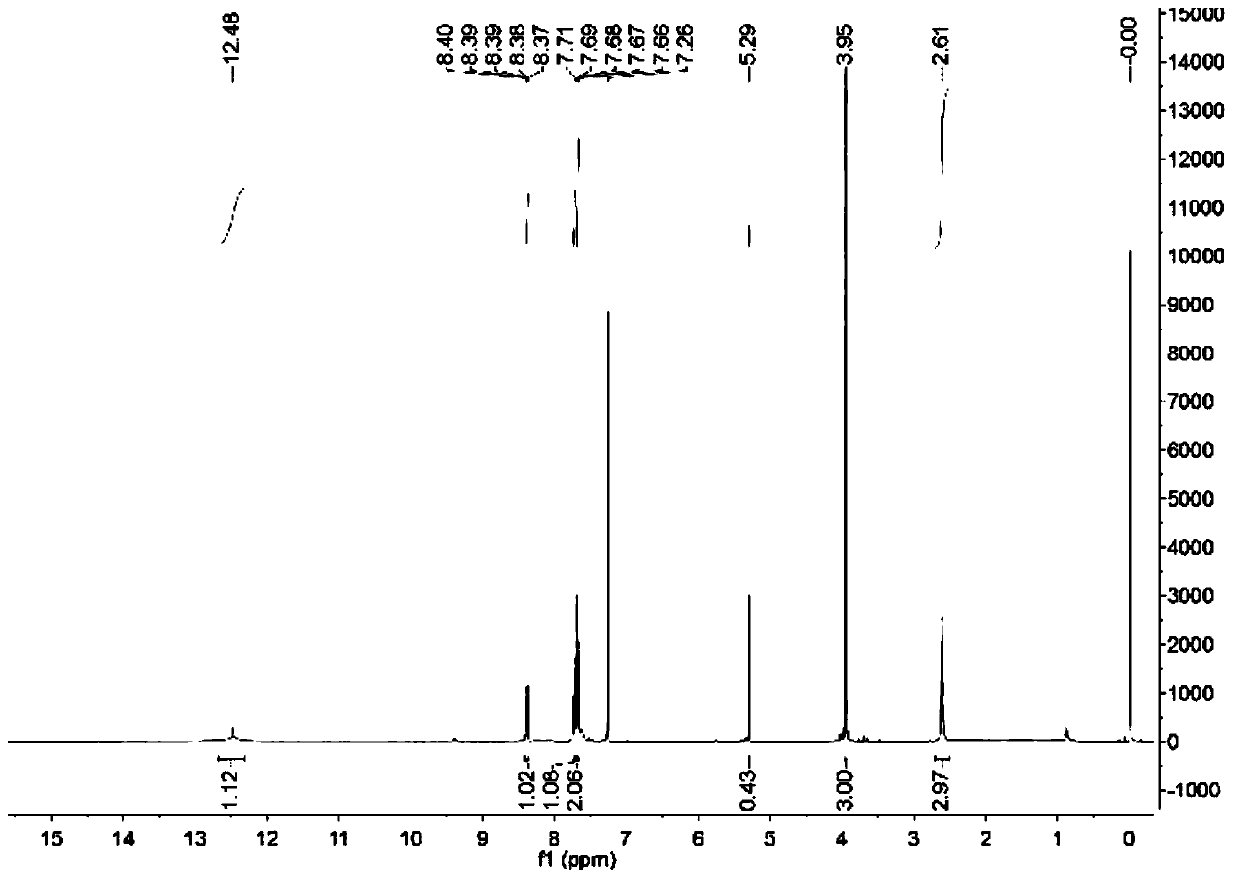
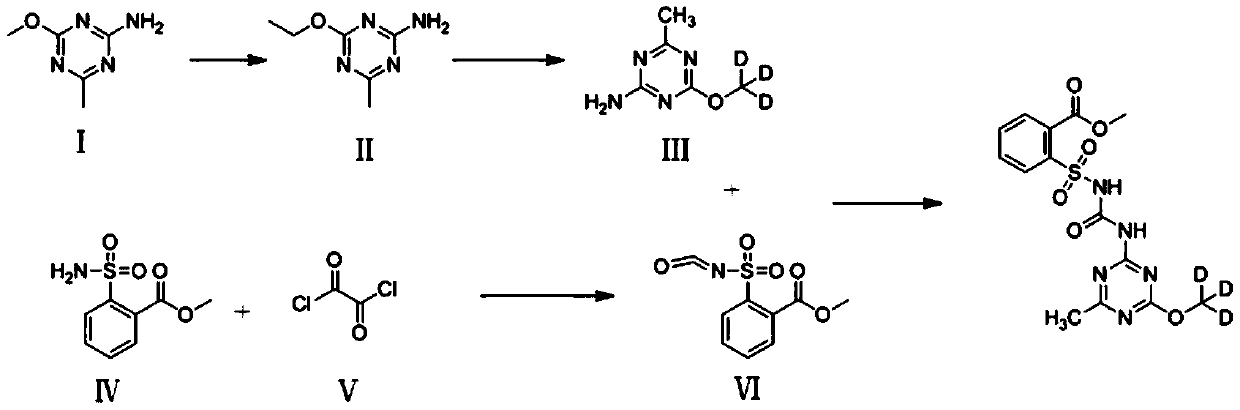
Synthetic process of metsulfuron-methyl-D3
A synthesis process, the technology of metsulfuron-methyl, is applied in the field of synthesis technology of metsulfuron-methyl-D3, can solve the problems of high requirements for equipment and operation, difficult to prepare in small quantities, and high synthesis cost, and achieves low cost and easy availability of raw materials. , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The synthetic technique of embodiment 1 metsulfuron-D3
[0042] first step:
[0043]
[0044] Add 5.0 g of sodium ethoxide (73.4 mmol) into 15 ml of absolute ethanol, stir to dissolve, add 976 mg of compound Ⅰ (7.0 mmol), stir at room temperature overnight, and obtain 920 mg of a light yellow solid by column chromatography, ESI-MS: 155[M+1]. Compound Ⅱ, yield 85.7%.
[0045] Step two:
[0046]
[0047] Preparation of tetrahydrofuran solution of deuterated sodium methoxide: add 2 ml of deuterated methanol to 20 ml of anhydrous THF, add 2 g of sodium metal, heat at 50 ° C, and react overnight under nitrogen protection. After 2 hours of reaction, stop heating and add 20 ml of anhydrous Dilute with water THF, remove the sodium block, set aside. A tetrahydrofuran solution of sodium deuterated methoxide was obtained, which contained 49.2 mmol of sodium deuterated methoxide.
[0048] Take 200mg of compound II (1.3mmol) and add it to the above solution, reflux for 5h,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com