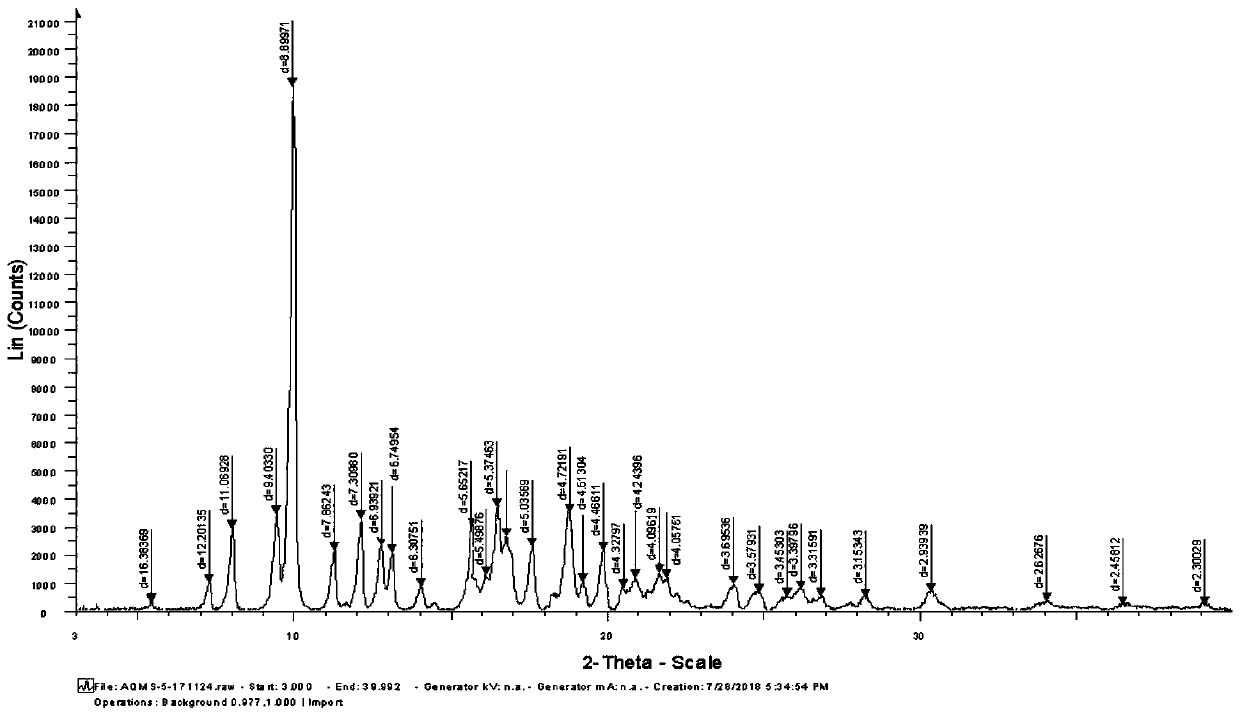
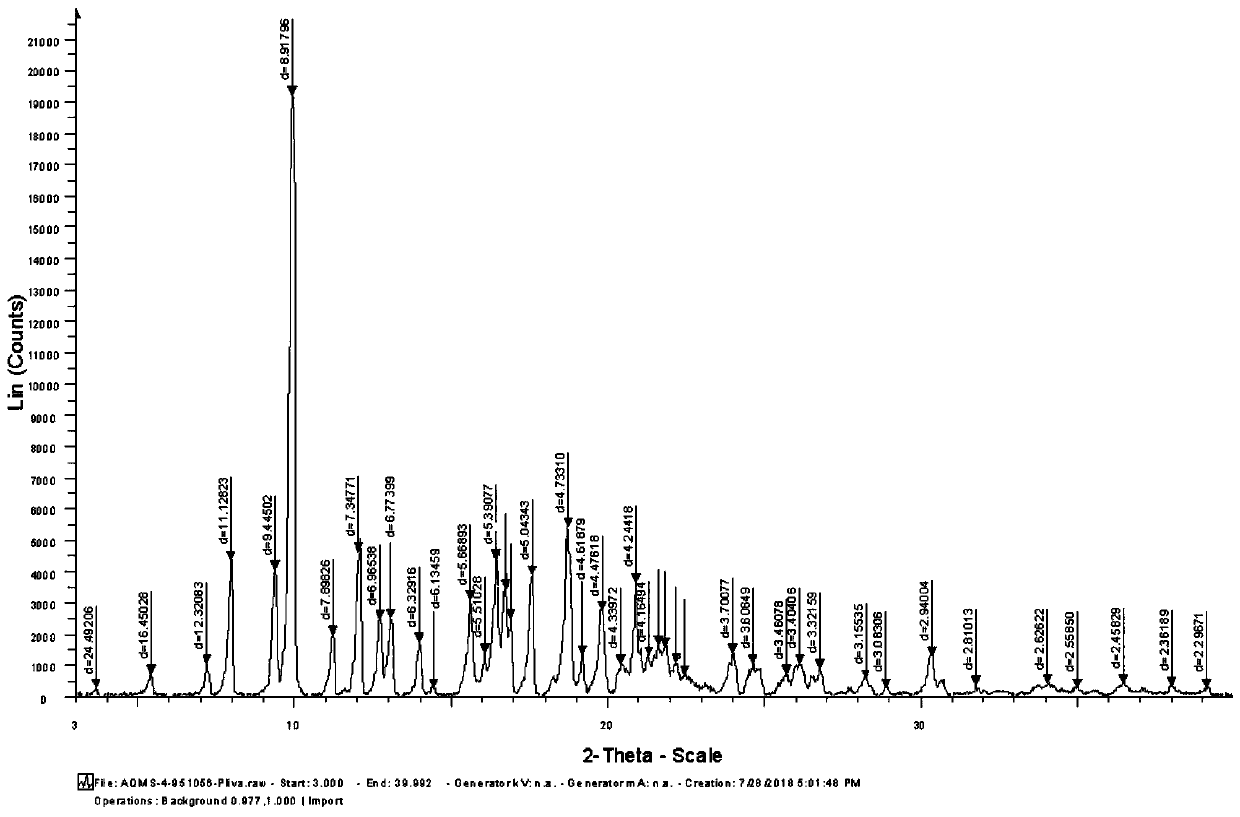
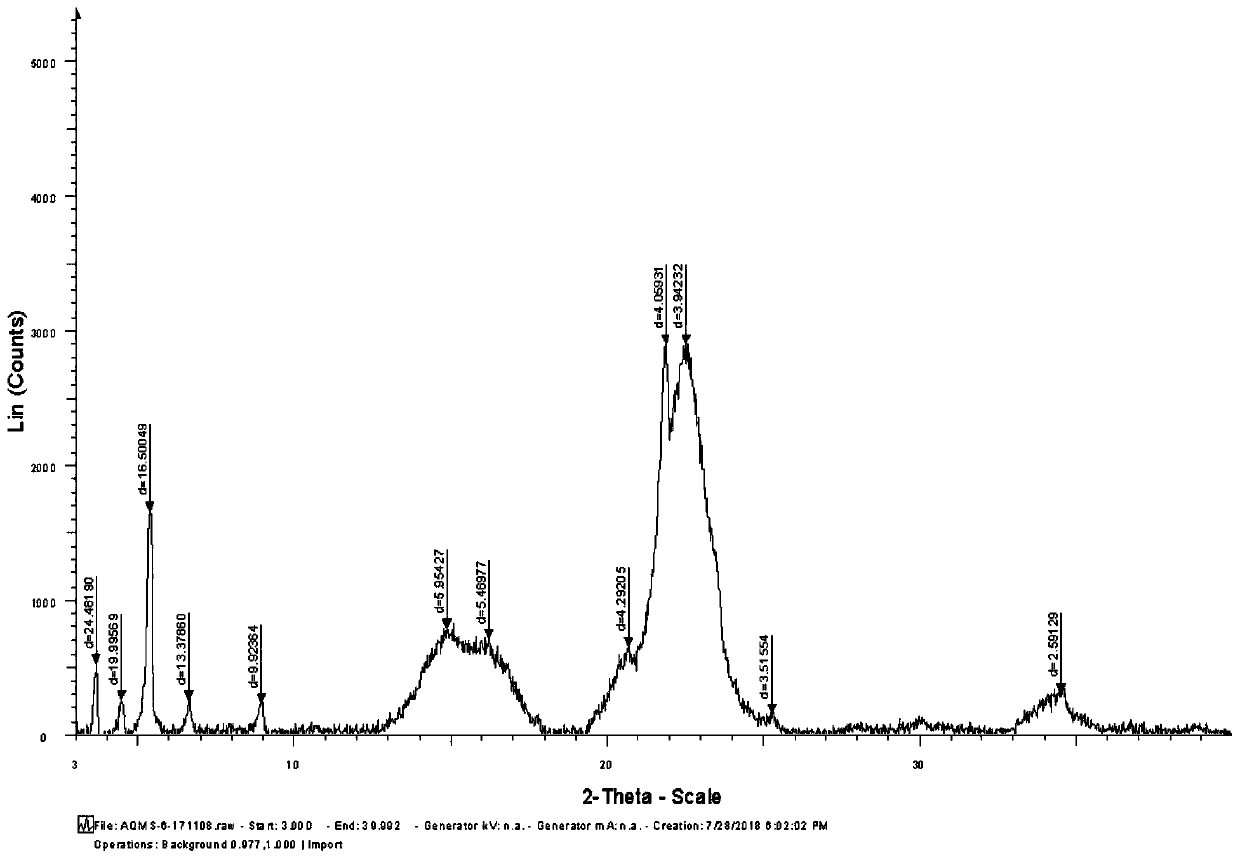
Preparing method of azithromycin capsule
A technology for azithromycin and capsules, which is applied in the field of preparation of azithromycin capsules, can solve problems such as easily destroyed crystal forms, uneven mixing of azithromycin granules, and unstable granulation. overcoming unstable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The present embodiment provides a kind of preparation method of azithromycin, and this preparation method comprises the following steps:
[0033] (1) Weigh 250.0g azithromycin, 80.0g microcrystalline cellulose, 2.0g sodium lauryl sulfate, and sieve the microcrystalline cellulose with a 40-mesh sieve to remove caking;
[0034] (2) Add azithromycin, microcrystalline cellulose, and sodium lauryl sulfate into a wet mixing granulator and stir for 3 minutes under the condition of 35 r / min;
[0035] (3) Add 42.0g of purified water into the wet granulator, cut for 55s under the condition of 50r / min, and make soft material;
[0036] (4) Add the soft materials into the oscillating granulator for granulation to obtain wet granules, and place the wet granules in a fluidized bed dryer to dry, the drying temperature is 45±5°C, and the drying time is 10min;
[0037] (5) The dried particles are sized by a 0.75mm aperture sieve;
Embodiment 2
[0040] The present embodiment provides a kind of preparation method of azithromycin, and this preparation method comprises the following steps:
[0041] (1) Weigh 250.0g azithromycin, 50.0g microcrystalline cellulose, and 1.5g sodium lauryl sulfate, and remove the agglomeration by sieving the microcrystalline cellulose with a 40-mesh sieve;
[0042] (2) Add azithromycin, microcrystalline cellulose, and sodium lauryl sulfate into a wet mixing granulator and stir for 3 minutes under the condition of 20-50r / min;
[0043] (3) Add 37.7g of purified water into the wet granulator, cut for 55s under the condition of 45r / min, and make soft material;
[0044] (4) Add the soft materials into the oscillating granulator for granulation to obtain wet granules, and place the wet granules in a fluidized bed dryer to dry, the drying temperature is 45±5°C, and the drying time is 10min;
[0045] (5) The qualified particles after drying are sized by a sieve with an aperture of 0.75 mm;
[0046...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com