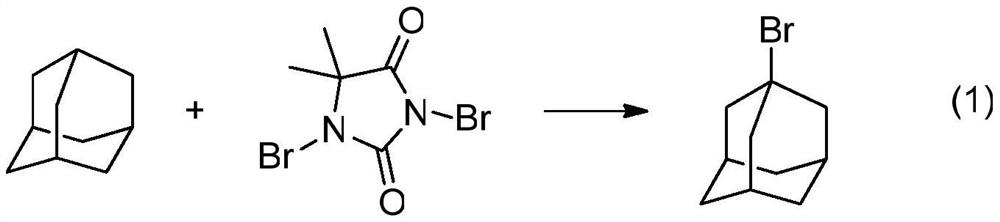
A kind of preparation method of 1-bromoadamantane
A technology of adamantane and bromination, applied in the field of preparation of 1-bromoadamantane, which can solve the problems of serious environmental pollution, complicated operation, and large equipment corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
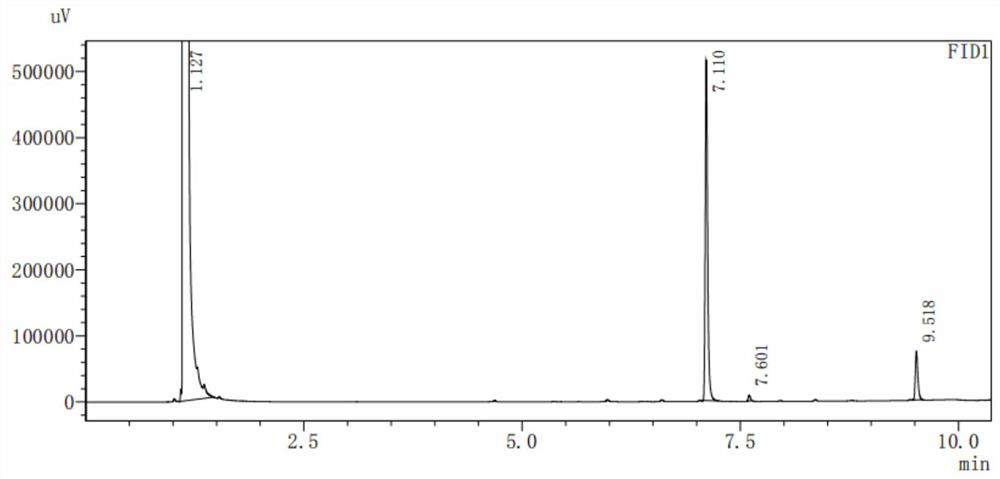
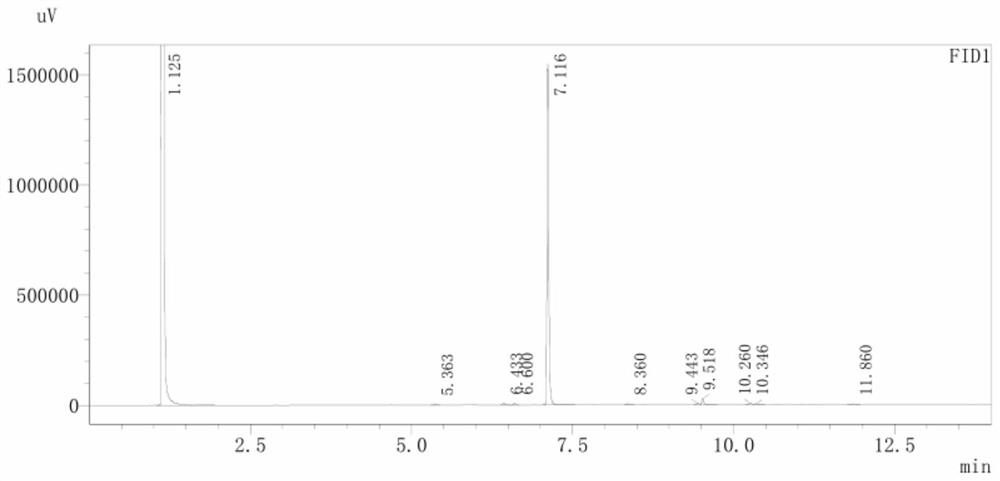
Image
Examples
Embodiment 1
[0025] Add 1.36g of adamantane (0.01mol), 1.43g (0.005mol) of dibromohydantoin (divided into 3 batches) into a 50ml three-necked flask, then add 25ml of dichloromethane, stir at room temperature for 30 minutes, and heat to the boiling point °C reflux. After reacting for 24 hours, turn off the heating and stirring, cool to room temperature, add saturated sodium bisulfite solution in an ice bath until the bromine yellow color disappears, stir for 15 minutes, remove the precipitated solid by filtration, separate the filtrate to obtain an organic phase, wash with 100 mL of water three times, dry, and concentrate to obtain crude product. The crude product was recrystallized from methanol to obtain 1.47g of white 1-bromoadamantane crystals with a yield of 68%. The melting point is 115.2-117.3°C. Replacement examples 1-1 to 1-8:
[0026] The preparation method is the same as in Example 1, the difference is that the type of solvent is adjusted to test its influence on the reaction,...
Embodiment 2
[0032] Add 1.36g of adamantane (0.01mol), 1.43g (0.005mol) of dibromohydantoin (divided into 3 batches) into a 50ml three-necked flask, then add 25ml of chloroform, stir at room temperature for 30 minutes, and heat to 30°C reflow. After reacting for 24 hours, turn off the heating and stirring, cool to room temperature, add saturated sodium bisulfite solution in an ice bath until the bromine yellow color disappears, stir for 15 minutes, remove the precipitated solid by filtration, separate the filtrate to obtain an organic phase, wash with 100 mL of water three times, dry, and concentrate to obtain crude product. The crude product was recrystallized from methanol to obtain 0.94g off-white 1-bromoadamantane crystals with a yield of 44%. The melting point is 116.0-116.8°C. Replacement examples 2-1 to 2-15:
[0033] The preparation method is the same as in Example 2, the difference is that the reaction temperature, reaction time, solvent (chloroform) usage amount, adamantane (1...
Embodiment 3
[0037] Embodiment 3: amplification reaction (5 times)
[0038] Add 6.81g of adamantane (0.05mol), 14.30g (0.05mol) of dibromohydantoin (divided into 3 batches) into a 250ml three-necked flask, then add 125ml of chloroform, stir at room temperature for 30 minutes, and heat to 65°C reflow. After reacting for 30 hours, turn off the heating and stirring, cool to room temperature, add saturated sodium bisulfite solution in an ice bath until the bromine yellow color disappears, stir for 15 minutes, remove the precipitated solid by filtration, separate the filtrate to obtain an organic phase, wash with 500 mL of water three times, dry, and concentrate to obtain crude product. The crude product was recrystallized from methanol to obtain 9.61 g of off-white crystals of 1-bromoadamantane with a yield of 89%. The melting point is 114.6-115.0°C.
[0039] Can draw in conjunction with above embodiment:
[0040] 1. The present invention uses 1,3-dibromo-5,5-dimethylhydantoin as a bromina...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com