Immune cells capable of clearing latent infections in vivo, preparation method and application thereof
A technology of immune cells and cells, applied in the application field of preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1. Preparation of SIET cells
[0058] 1. Isolation of Peripheral Blood Lymphocytes
[0059] (1) One blood draw volume: 150ml, anticoagulated with heparin. 150ml of whole blood can normally get 1.5-2×10 8 cells, can plant a 75cm 2 Culture flask; the peripheral blood treated with heparin anticoagulation was divided into centrifuge tubes, centrifuged at 1800rpm for 15min, and the supernatant was discarded;
[0060] (2) Dilute the blood: AIMV culture medium is used to dilute the blood to make a lymphocyte suspension;
[0061] (3) 25ml of lymphocyte suspension was carefully placed on 12.5ml of lymphocyte separation medium, centrifuged at 1800rpm for 15min, and the lifting speed was adjusted to the lowest;
[0062] (4) Take the upper part of the plasma and keep it in a refrigerator at 4°C;
[0063] (5) Take the lymphocyte layer, place it in a 50ml centrifuge tube, add AIMV medium to a total volume of 50ml, centrifuge at 1800rpm for 5min;
[0064] (6) Discard ...
Embodiment 2
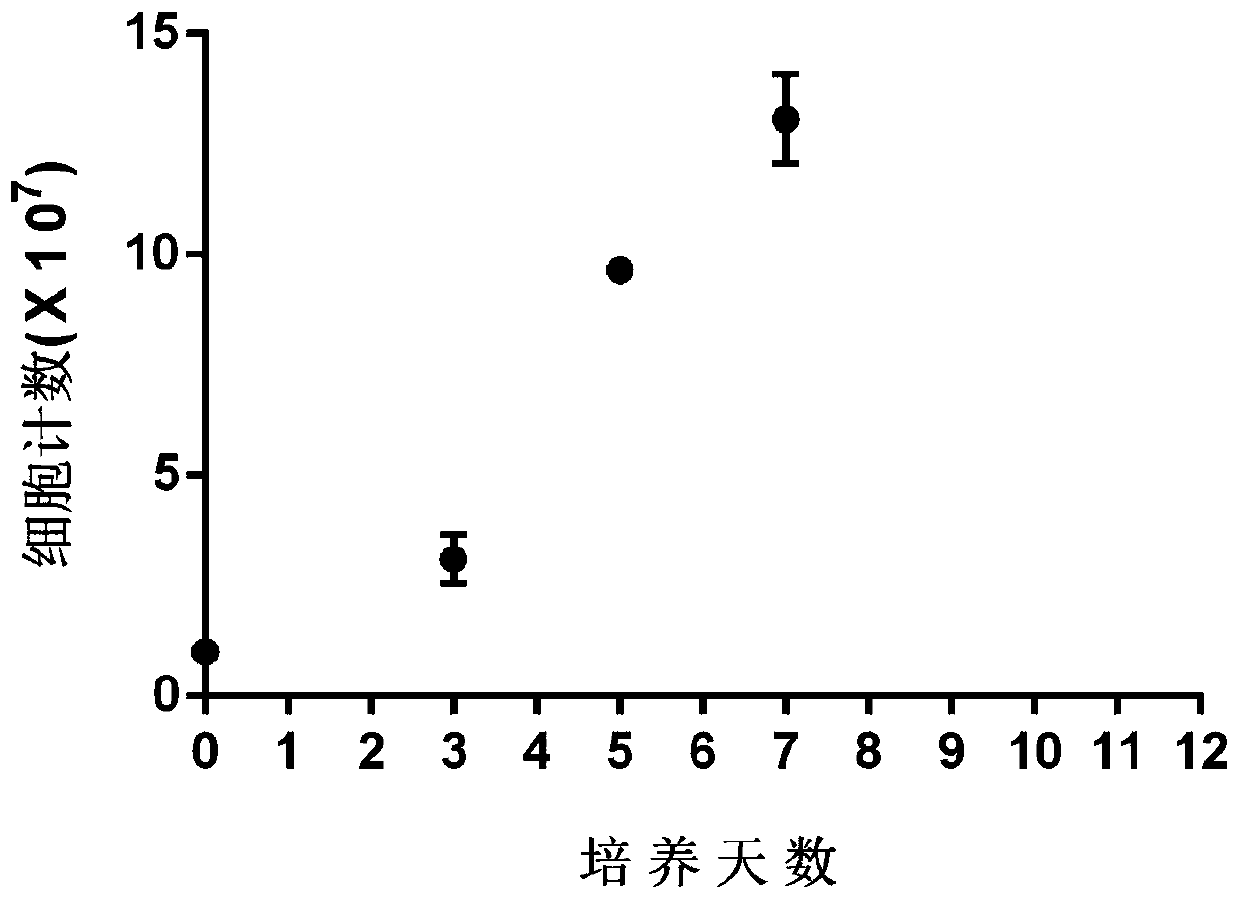
[0101] Example 2.Detection of SIET cell expansion ability
[0102] Adopt the method for embodiment 1 to cultivate, and take the SIET cells that culture time is the 0th, 3, 5, 7, 9, 12 days, detect cell proliferation situation, as figure 1 . Depend on figure 1 It can be seen that the proliferation of SIET cells cultured by the method of the present invention is better.
Embodiment 3
[0103] Example 3.SIET cell viability detection
[0104] The method of Example 1 was used to culture, and 100 μl of cells cultured for 12 days was taken, and 100 μl of 0.4% trypan blue staining solution was added. The living cells were not stained, and the dead cells were stained blue. The results are shown in Table 1.
[0105] Table 1
[0106] sample 1 sample 2 sample 3 Percentage of Viable Cells 95.5% 97.1% 95.9%
[0107] It can be seen from Table 1 that the viability of the cells prepared by the present invention is greater than 95%, and the viability of the cultured SIET cells is better.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com