Preparation method of triclopyr butoxyethyl ester
A technology of butoxyethyl ester and butoxyethyl hydroxyacetate is applied in the field of preparation of triclopyr butoxyethyl ester, which can solve the problem of low content of final product, expensive recovery of acid binding agent and easy generation of isomers and other problems, to achieve the effect of high product yield, cheap raw materials and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
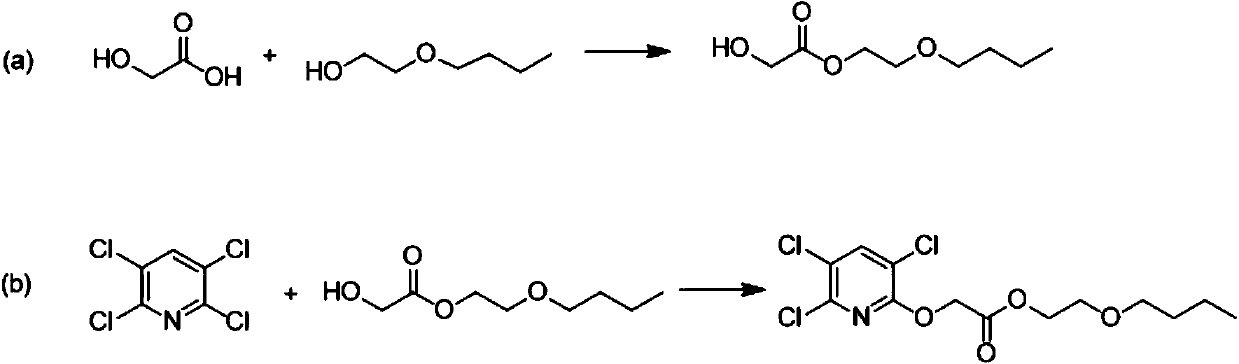
[0025] The invention provides a preparation method of triclopyr butoxyethyl ester, comprising the steps of:
[0026] (a) glycolic acid and ethylene glycol monobutyl ether undergo an esterification reaction to obtain butoxyethyl glycolate;
[0027] (b) Butoxyethyl glycolate is reacted with tetrachloropyridine to obtain the target product triclopyr-butoxyethyl.
[0028] Wherein, the reaction process of step (a) is carried out in the presence of a catalyst, and the catalyst is an acidic catalyst, and the acidic catalyst can be selected from general acidic catalysts, solid acids, heteropolyacids or solid superacids, preferably sulfuric acid or p-toluenesulfonic acid.
[0029] Further, the mass ratio of catalyst to glycolic acid is 0.1%-5%:1, preferably 4%:1.
[0030] Wherein, in the present invention, the reaction temperature of step (a) is controlled at 80-110°C, the reaction temperature of step (b) is controlled at 60-110°C, preferably, the reaction temperature of step (b) is c...
Embodiment 1
[0044] Add 500g of toluene, 326.3g of ethylene glycol monobutyl ether, and 7.5g of sulfuric acid into a 2000mL reaction bottle, and add 250g of 70% glycolic acid dropwise at 80-85°C. Pour into water, wash with sodium bicarbonate until the water phase is neutral, separate phases, concentrate and recover toluene, and then distill to obtain 377.1 g (yield 93%) of butoxyethyl glycolate with a content of 96.8%;
Embodiment 2
[0046] Add 500g of cyclohexane, 326.3g of ethylene glycol monobutyl ether, and 7.5g of sulfuric acid into a 2000mL reaction flask, raise the temperature to 80°C and add 250g of 70% glycolic acid dropwise. Pour into water, wash with sodium bicarbonate until the water phase is neutral, separate phases, concentrate and recover cyclohexane, and then distill to obtain 364.9 g (yield 90%) of butoxyethyl glycolate with a content of 93%;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com