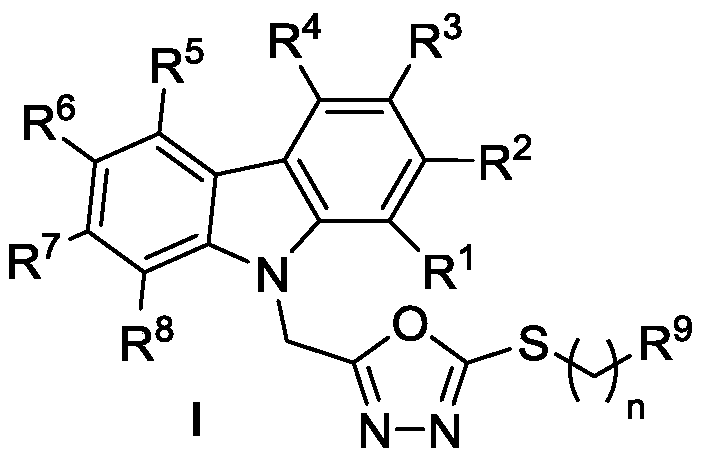
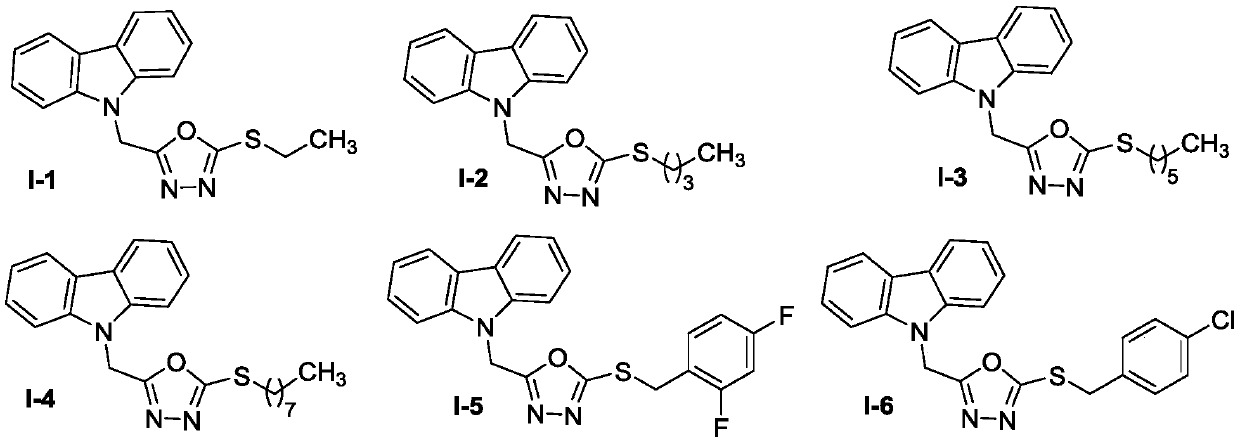
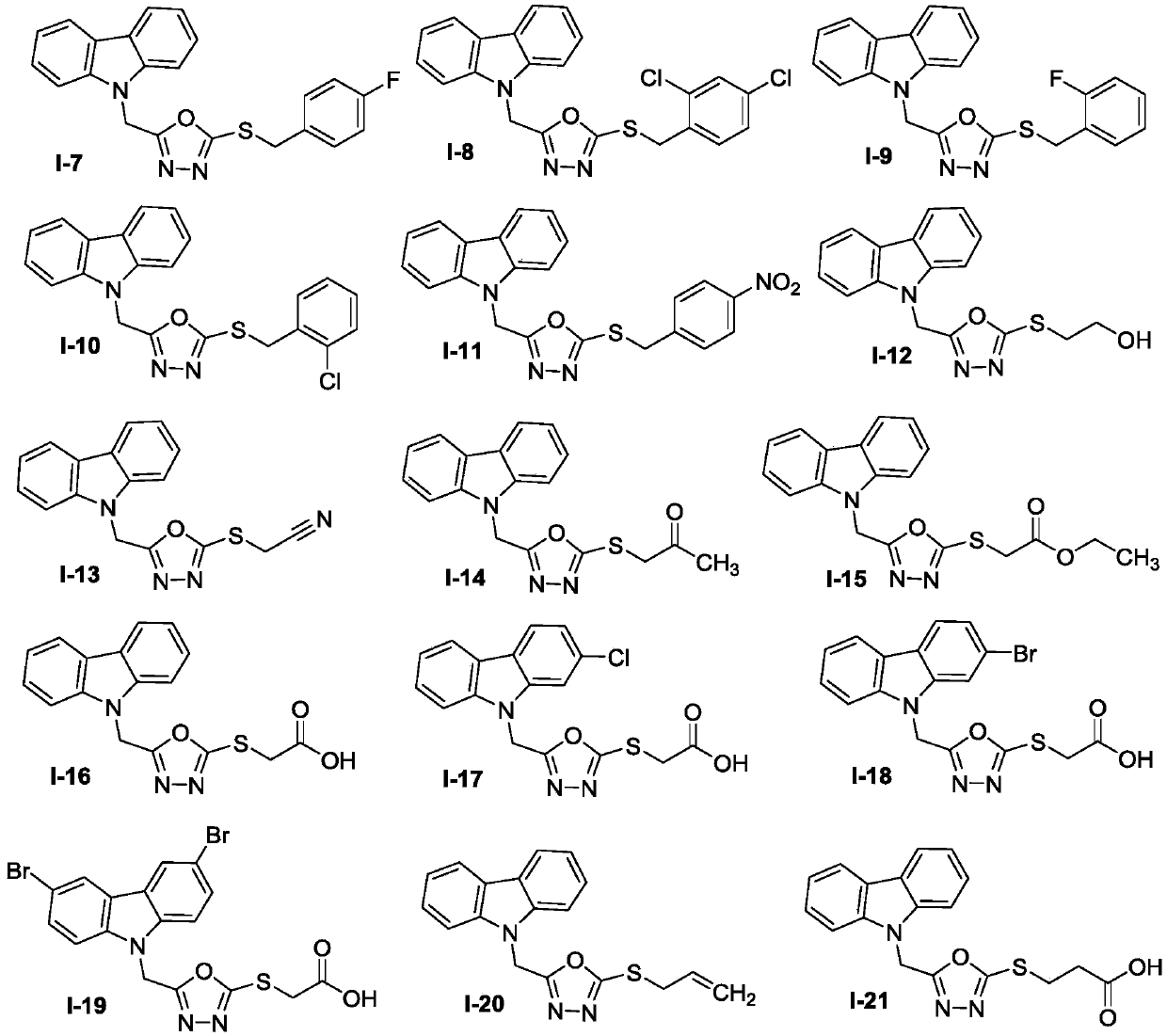
Carbazole oxadiazole conjugates, and preparation method and application thereof
A technology of oxadiazole and carbazole, which is applied in the field of chemical synthesis, can solve the problems of poor solubility and limited application of carbazole compounds, and achieve the effects of solving drug resistance, simple preparation of raw materials, and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the preparation of intermediate II-1:
[0043]
[0044]Stir N,N-dimethylformamide (100 mL) containing carbazole (1.000 g, 5.980 mmol) and cesium carbonate (2.530 g, 7.780 mmol) at 85 °C for 30 minutes, cool slightly, then add 2-bromo Ethyl acetate (1.300g, 7.780mmol), continue to stir the reaction at 85°C, track by thin-layer chromatography until the reaction is complete, pour the reaction mixture into ice water to precipitate a solid, suction filter, wash and dry to obtain the intermediate II-1 (1.067g), yield: 70.5%.
Embodiment 2
[0045] Embodiment 2: the preparation of intermediate II-2:
[0046]
[0047] N,N-dimethylformamide (100 mL) containing 2-bromocarbazole (1.000 g, 4.063 mmol) and cesium carbonate (1.721 g, 5.282 mmol) was stirred at 85°C for 30 minutes, cooled slightly, and then added 2-Ethyl bromoacetate (0.882g, 5.282mmol), continue to stir the reaction at 85°C, track by thin layer chromatography until the reaction is complete, pour the reaction mixture into ice water to precipitate a solid, suction filter, wash and dry, Intermediate II-2 (1.017g) was obtained, yield: 75.6%.
Embodiment 3
[0048] Embodiment 3: the preparation of intermediate II-3:
[0049]
[0050] N,N-dimethylformamide (100 mL) containing 2-chlorocarbazole (1.000 g, 4.974 mmol) and cesium carbonate (2.1071 g, 6.467 mmol) was stirred at 85°C for 30 minutes, cooled slightly, and then added 2-Ethyl bromoacetate (1.080g, 6.467mmol), continue to stir the reaction at 85°C, track by thin-layer chromatography until the reaction is complete, pour the reaction mixture into ice water to precipitate a solid, suction filter, wash and dry, Intermediate II-3 (1.104 g) was obtained with a yield of 77.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com