Fluoroalkylation method of steroid 17 beta-thiocarboxylic acid
A technology of thiocarboxylic acid and fluoroalkylation, which is applied in the production of steroids, organic chemistry, and bulk chemicals, and can solve the problems of low and high content of fluoroformation products, and uncontrollable reaction conditions. Achieve the effect of reducing the residue of impurities and reducing the difficulty of refining
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
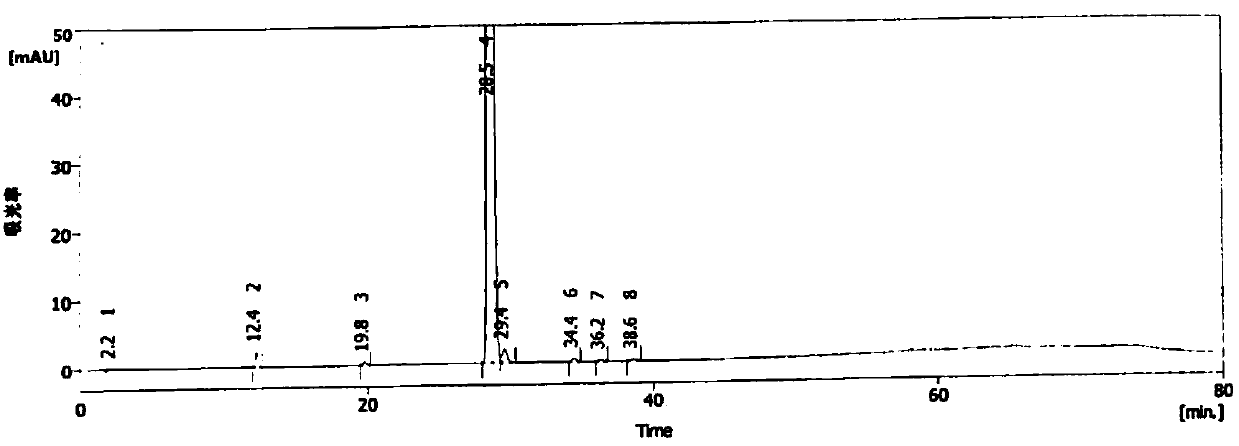
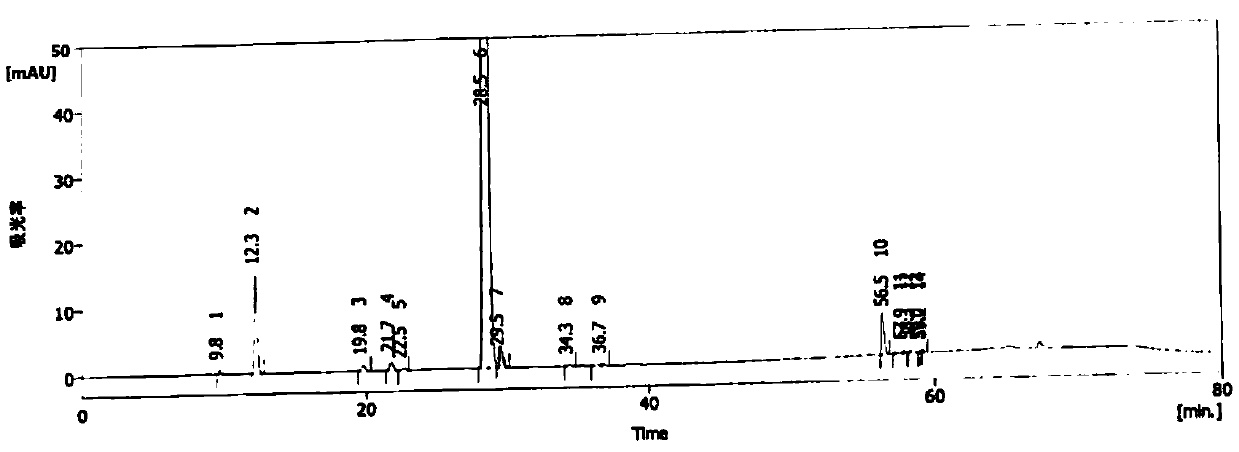
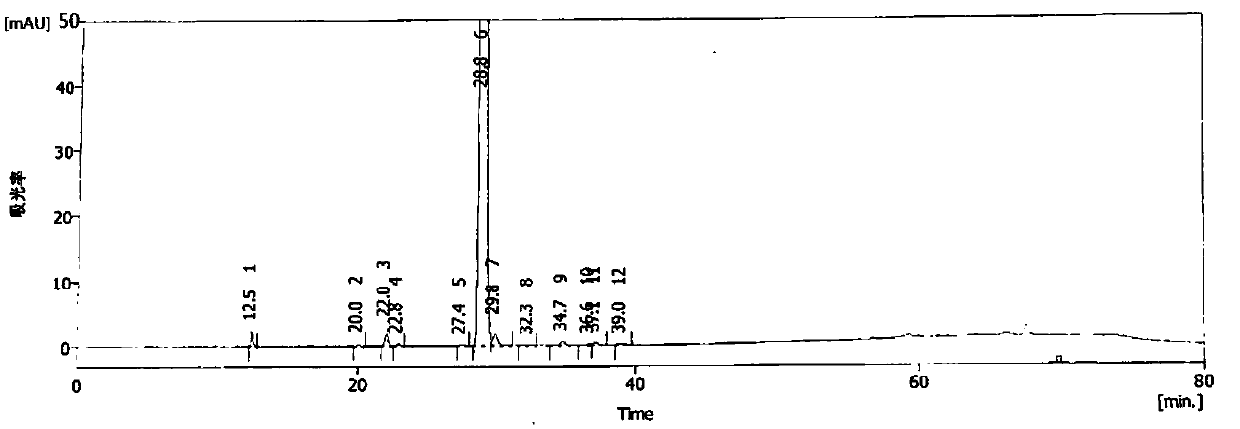
Image
Examples
Embodiment 1
[0063]
[0064] Add 50ml of dimethylacetamide and 10g of compound 1 into a four-necked reaction flask, cool down to -5°C, add 2.9g of sodium sulfite, and then dropwise add 2.0ml of bromofluoromethane. After the reaction is complete, add 500ml of purified water to precipitate a solid. 10.4g of crude compound 2 was obtained by filtration. The detected compound 2 content was 98.8%, the mercapto oxide content was 0.38%, the haloalkylated impurities were 0.19%, and the disulfide compound was not detected. The solid compound 2 crude product obtained was recrystallized by adding absolute ethanol , suction filtration, and drying to obtain 10.1 g of compound 2, with a content of 99.7%.
Embodiment 2
[0068]
Embodiment 2-1
[0070] Add 100ml of acetonitrile and 10g of compound 3 into a four-necked reaction flask, cool down to -15°C, add 4.0g of potassium carbonate and 1.0g of potassium sulfite, then dropwise add 2.0ml of fluoroiodomethane, after complete reaction, add 500ml of purified water to precipitate As a solid, 10.3 g of crude compound 4 was obtained by suction filtration, with a content of 98.8%, a content of mercapto oxides of 0.40%, haloalkylated impurities of 0.21%, and no disulfide compounds were detected. The obtained crude solid compound 4 was recrystallized by adding absolute ethanol, suction filtered, and dried to obtain 9.8 g of refined compound 4 with a content of 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com