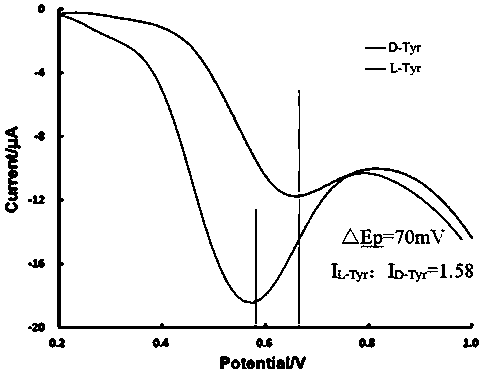
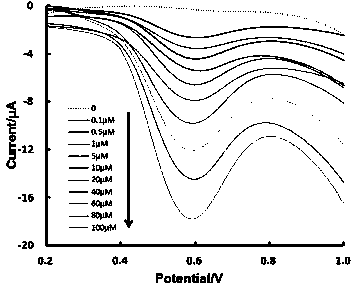
Electrochemical chiral sensor constructed by reduced graphene composite material
A composite material, graphene technology, applied in the direction of material electrochemical variables, scientific instruments, analytical materials, etc., can solve problems such as ineffectiveness and toxicity, and achieve the effect of wide electrochemical window, low cost, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
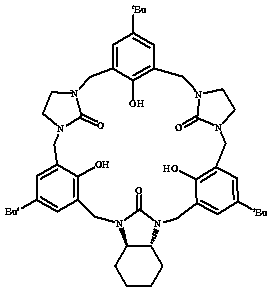
[0028] Embodiment 1: a kind of reduced graphene composite material, is with attached figure 1 The chiral macrocyclic compound shown is obtained after functionalized reduction of graphene. The functionalization method is as follows: dissolve the reduced graphene in dimethyl sulfoxide, ultrasonicate for 2 hours at room temperature to obtain a suspension, and pour it into the suspension In the turbid liquid, add the macrocyclic compound with the mass ratio of reduced graphene: macrocyclic compound=1:2, control the mass concentration of the reduced graphene in the system to be 0.25mg / mL, then stir at room temperature for 40h, and obtain the function of the macrocyclic compound. reduced graphene composites.
[0029] The reduced graphene is prepared by the following method: at room temperature, graphene oxide is placed in secondary water for ultrasonication for 2 hours to disperse it into graphene oxide sheets; then add ascorbic acid in a mass ratio of 1:12, and stir at room tempera...
Embodiment 2
[0035] Embodiment 2: a kind of reduced graphene composite material is with attached figure 1 The chiral macrocyclic compound shown is obtained after functionalized reduction of graphene. The functionalization method is as follows: dissolve the reduced graphene in dimethyl sulfoxide, ultrasonicate for 2 hours at room temperature to obtain a suspension, and pour it into the suspension In the turbid liquid, add the macrocyclic compound with the mass ratio of reduced graphene: macrocyclic compound=1:2.5, control the mass concentration of the reduced graphene in the system to be 0.3mg / mL, then stir at room temperature for 60h, and obtain the function of the macrocyclic compound. reduced graphene composites.
[0036] The reduced graphene is prepared by the following method: at room temperature, graphene oxide is placed in secondary water and ultrasonically charged for 2 hours to disperse it into graphene oxide sheets; then add ascorbic acid in a mass ratio of 1:15, and stir at room ...
Embodiment 3
[0042] Embodiment 3: a kind of reduced graphene composite material, is with attached figure 1 The chiral macrocyclic compound shown is obtained after functionalized reduction of graphene. The functionalization method is as follows: dissolve the reduced graphene in dimethyl sulfoxide, ultrasonicate at room temperature for 1 hour to obtain a suspension, and pour the suspension into In the turbid liquid, the mass ratio of reduced graphene:macrocyclic compound=1:1.5 is added to the macrocyclic compound, and the mass concentration of the reduced graphene in the control system is 0.2mg / mL, and then stirred at room temperature for 20h to obtain the function of the macrocyclic compound. reduced graphene composites.
[0043] The reduced graphene is prepared by the following method: at room temperature, graphene oxide is placed in secondary water for 1 h to be dispersed into graphene oxide sheets; then ascorbic acid is added at a mass ratio of 1:10, and stirred at room temperature for 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com