Preparation of garnet type solid electrolyte and secondary battery using garnet type solid electrolyte
A solid-state electrolyte, garnet-type technology, applied in the field of lithium ion batteries, can solve the problems of low load of electrode active material, volume change of electrode material, poor cycle stability, etc., and achieve wide electrochemical window, high safety and high stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
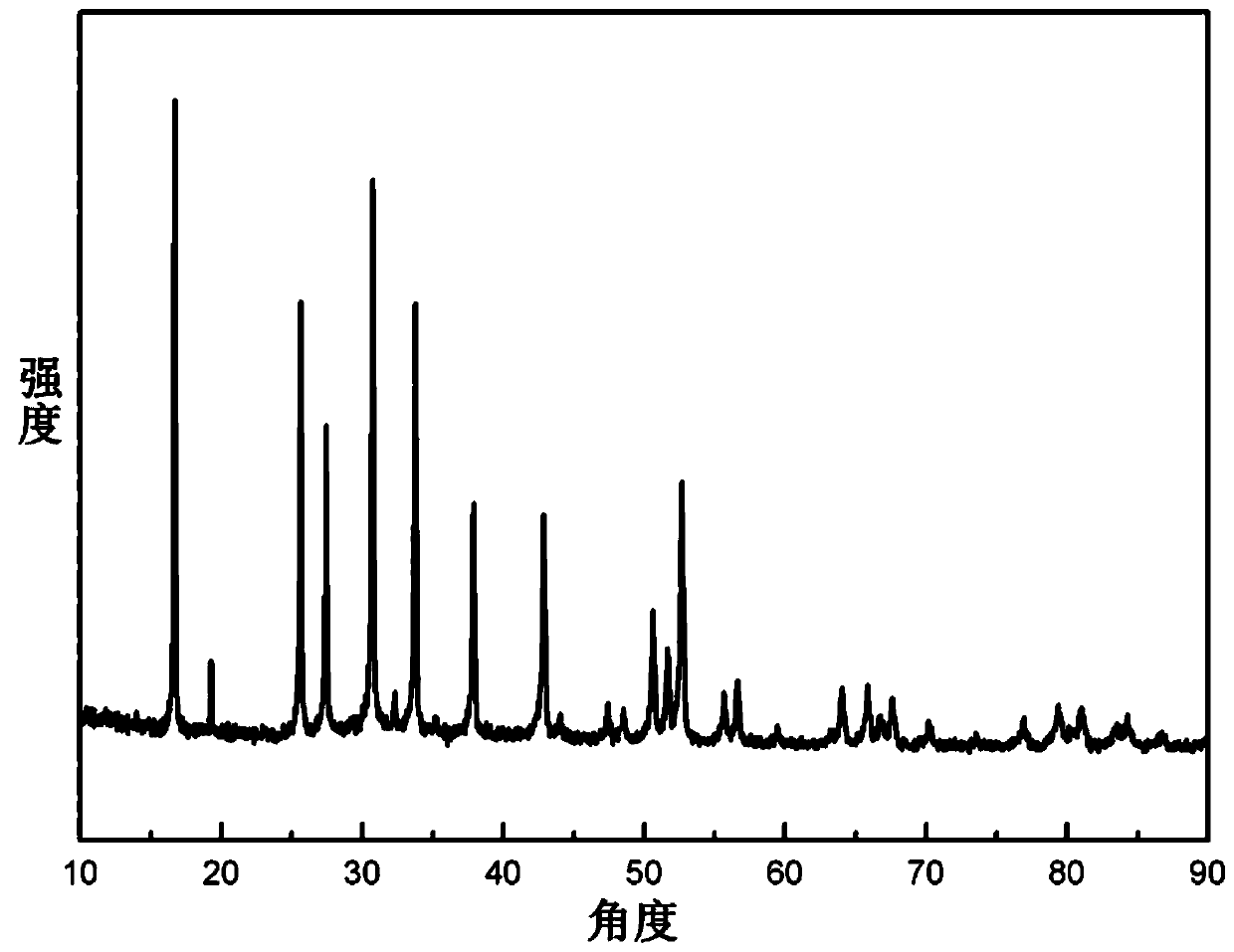
[0043]According to the characteristics of the sol-gel method, anhydrous lithium nitrate, lanthanum nitrate hexahydrate, and zirconium acetylacetonate are preferably used as metal sources for preparing LLZO. According to Li 7 La 3 Zr 2 o 12 Weigh anhydrous lithium nitrate (excess 10% to 20%), lanthanum nitrate hexahydrate, zirconium acetylacetonate, add a small amount of aluminum nitrate, dissolve in water to prepare a solution and stir evenly with a magnetic stirrer. The complexing agent citric acid was dissolved in water and magnetically stirred evenly, and several drops of 65% nitric acid were added to the citric acid solution. Add the complexing agent dropwise to the mixed liquid, heat and evaporate the solvent after the reaction is complete, the evaporation temperature is generally slightly lower than the boiling point of the solvent, so the evaporation temperature is preferably 80°C. As the solvent evaporated, a dark gel was obtained. The resulting gel was dried for ...
Embodiment example 2
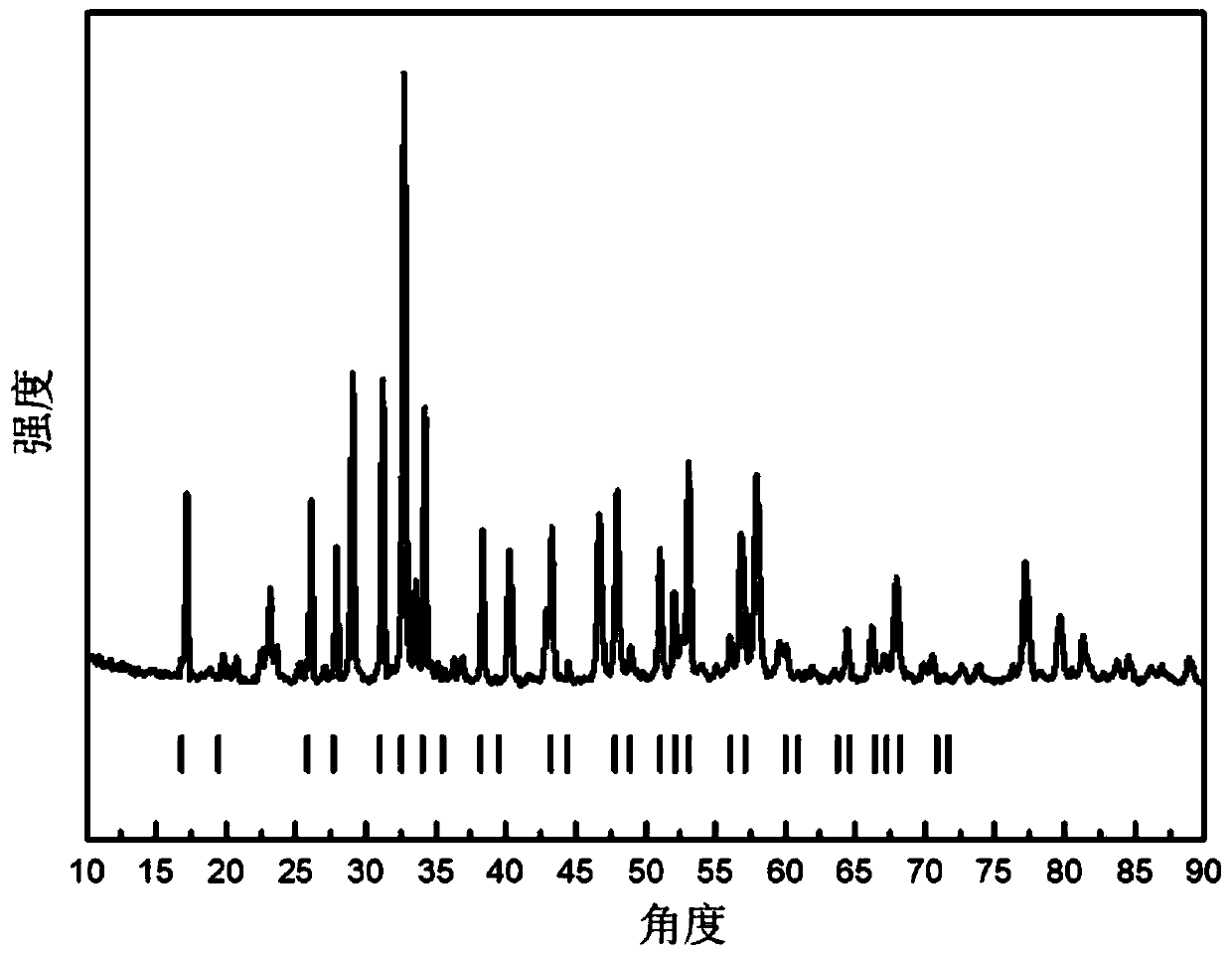
[0045] The buffer layer material preparation is similar to the LLZO preparation process. When the metal salt is completely dissolved, a certain amount of lithium iron phosphate powder is added to obtain a uniformly dispersed turbid liquid. The complexing agent citric acid is dissolved in water and magnetically stirred evenly, and several drops of 65% nitric acid are added to the citric acid solution. Add complexing agent dropwise to the mixed liquid to carry out complexation reaction. After the reaction is complete, heat and evaporate the solvent to dryness. The evaporation temperature is generally slightly lower than the boiling point of the solvent, so the evaporation temperature is preferably 80°C. As the solvent evaporated, a black gel was obtained. The resulting gel was dried for 48 hours in a blast drying oven to obtain a xerogel. The drying temperature at this stage is higher than the boiling point of water, preferably 100°C. After the dry gel was ground evenly, it ...
Embodiment example 3
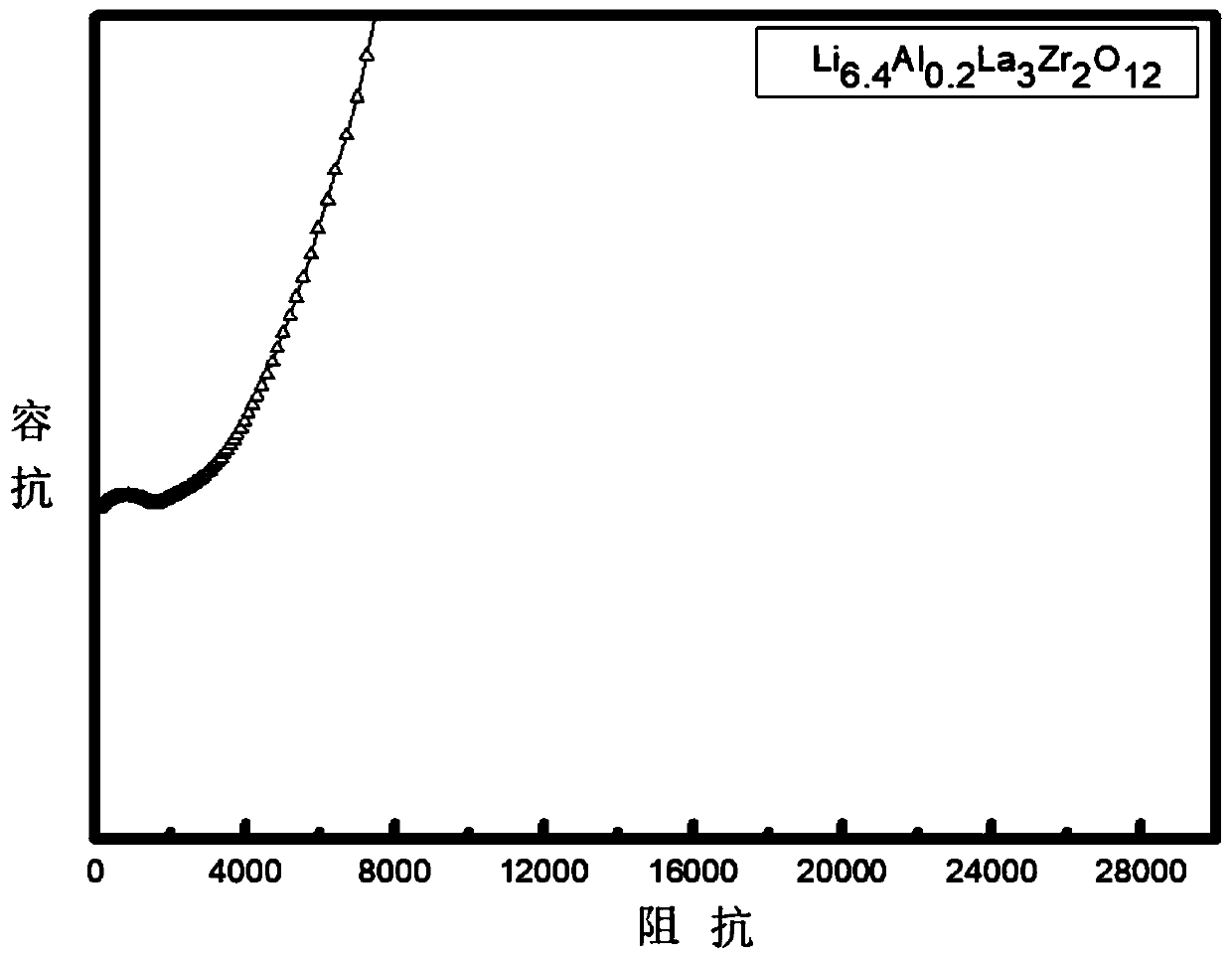
[0047] The conductivity test of inorganic solid electrolytes is generally carried out in the form of ceramic sheets, and metals, such as Au, are sputtered or evaporated on both ends of the ceramic sheets. This is used as a blocking electrode for testing, since Au + It has a certain blocking effect, so it presents an obvious capacitive reactance arc in the low frequency region of the impedance spectrum. The prepared ceramic sheet was polished, and after the surface of the LLZO ceramic sheet was plated with gold, the AC impedance test was carried out on the LLZO ceramic sheet. Using EIS AC impedance spectroscopy for testing. The frequency range is 0.1Hz ~ 1MHz, and the test temperature is room temperature. Spectrum such as image 3 It is shown that the as-prepared material has low electrical resistance and high ionic conductivity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com