A kind of polyarylsulfone polymer containing reduced phenazine structure and preparation method thereof
A polyarylsulfone and polymer technology is applied in the field of polyarylsulfone polymers containing a reduced phenolazine structure and the preparation thereof, and can solve the problems of difficult photoelectric activity, electrochemical stability, thermal stability, solubility and the like, Achieve the effect of easy processing into film, improved solubility, and broad development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation method of the polyarylsulfone polymer containing the reduced phenazine structure of the present invention comprises the following steps:
[0045]Put the difluoro monomer containing sulfone group, bisphenol A, reduced phenazine, catalyst potassium carbonate, solvent sulfolane or N-methylpyrrolidone, and water-carrying agent toluene into a three-stage tank equipped with nitrogen port, oil-water separator, and mechanical stirring. In a nitrogen atmosphere, stir and heat to 140-150°C to reflux the toluene for 3 hours, then use an oil-water separator to release toluene and water after fully carrying water, then raise the temperature to 200-220°C and stir for 3-8 hours to obtain To reduce the phenazine polyarylsulfone polymer, the reaction formula is (i):
[0046]
[0047] In the formula, n is the degree of polymerization, 0<m≤1 represents the copolymerization ratio, and Ar is one of the formulas (a) to (d):
[0048]
[0049] In formula (c), X is select...
Embodiment 1
[0056] Embodiment 1: the preparation of polymer P1
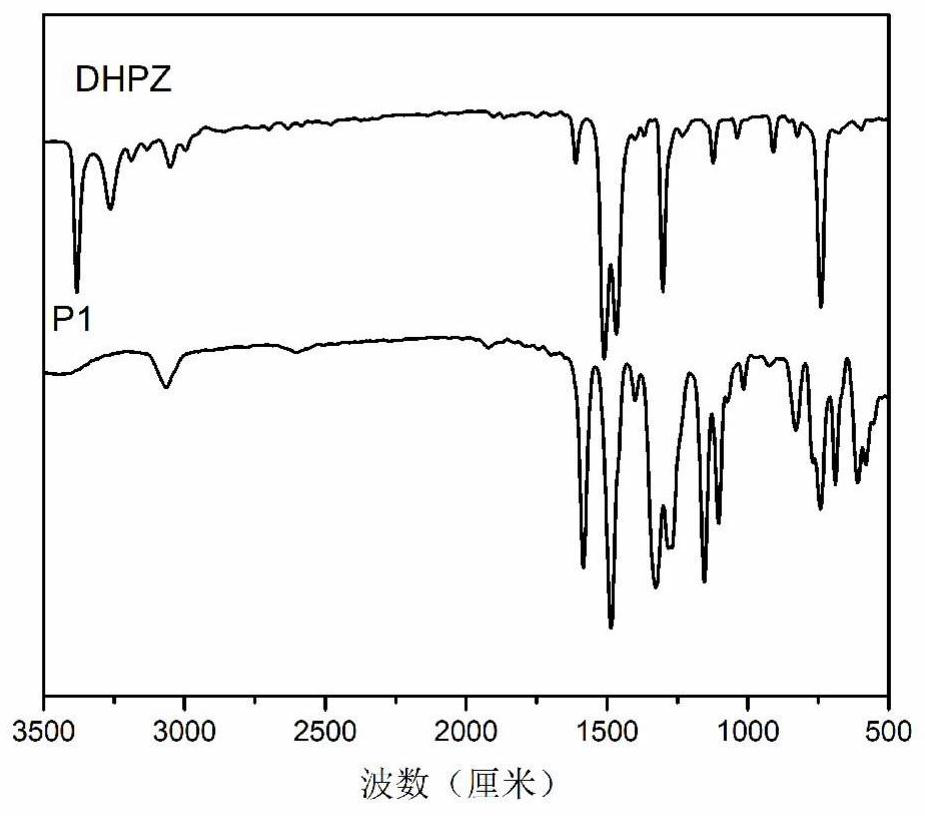
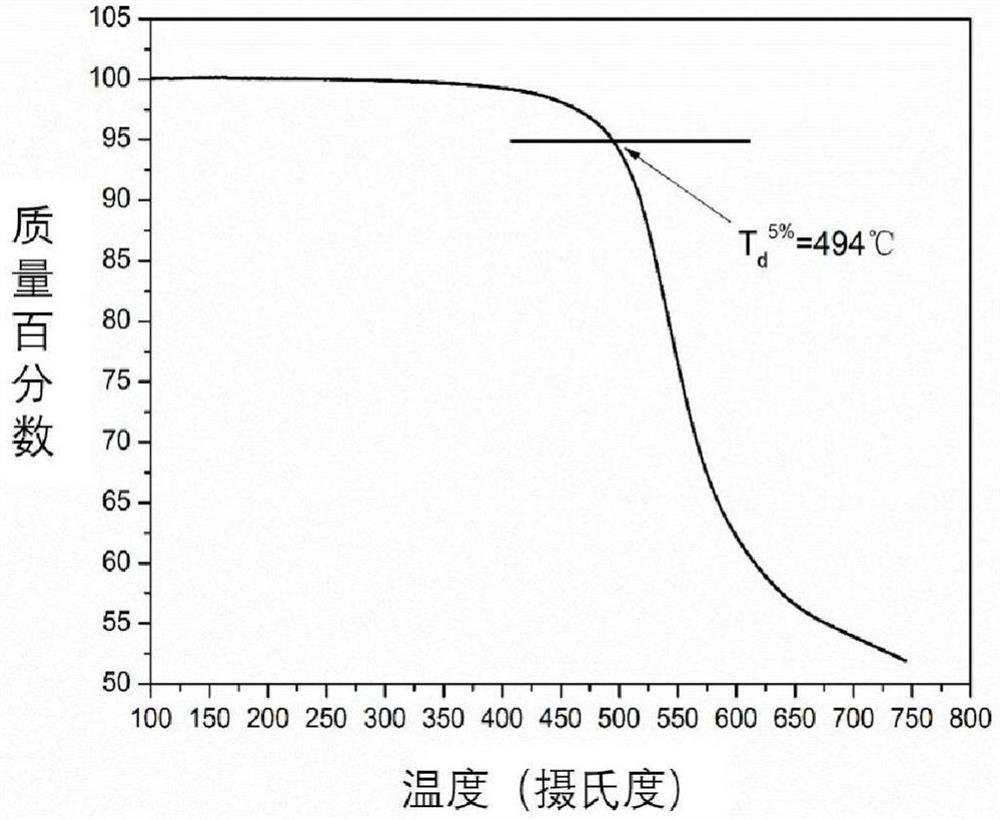
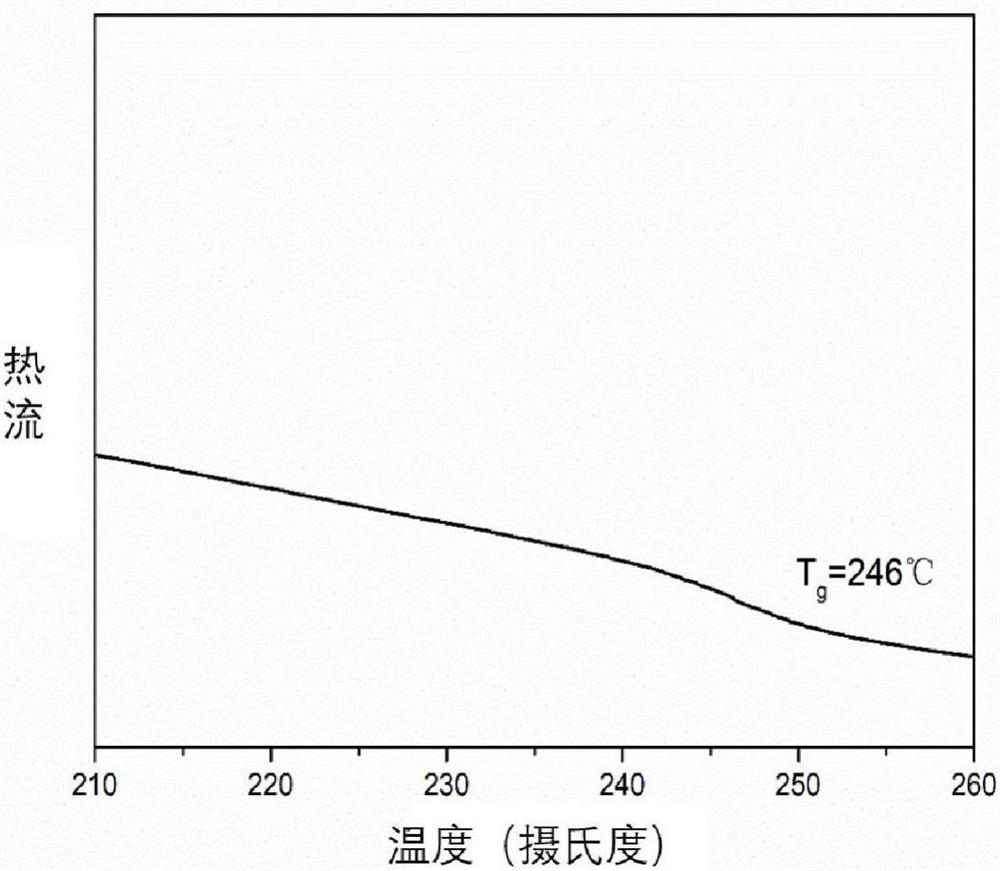
[0057] Put reduced phenazine (3.685g, 20mmol), 4,4'-difluorodiphenyl sulfone (5.334g, 20mmol), catalyst potassium carbonate (5.521g, 40mmol), 26mL NMP, 20mL water-carrying agent toluene into a nitrogen ventilator In a 100mL three-necked flask with an oil-water separator and a mechanical stirrer, stir and heat in a nitrogen atmosphere until the toluene refluxes for 3 hours. After fully carrying water, release toluene and water with an oil-water separator. Then raise the temperature to 200°C and stir the reaction for 8h. After the reaction was completed, the solution was poured into 800 mL of cold water with stirring. Use a tissue pulverizer to grind it into powder, filter under reduced pressure, collect the solid precipitate, and then boil and wash with hot water (5 times, 800 mL each) and ethanol (3 times, 300 mL each), filter and collect, and put it in an oven Dry at 80°C for 10 hours. An orange-yellow polymer powder (7....
Embodiment 2
[0061] Embodiment 2: the preparation of polymer P1-50%
[0062] Reduced phenazine (1.842g, 10mmol), 4,4'-difluorodiphenylsulfone (5.334g, 20mmol), bisphenol A (2.284g, 10mmol), catalyst potassium carbonate (5.521g, 40mmol), 34mL sulfolane Put 20mL of toluene with water agent into a 100mL three-necked flask equipped with a nitrogen port, an oil-water separator, and a mechanical stirrer, stir and heat in a nitrogen atmosphere until the toluene refluxes for 3 hours, and release toluene and water with an oil-water separator after fully carrying water . Then raise the temperature to 220°C and stir the reaction for 4h. After the reaction was completed, the solution was poured into 800 mL of cold water with stirring. Use a tissue pulverizer to grind it into powder, filter under reduced pressure, collect the solid precipitate, and then boil and wash with hot water (5 times, 800 mL each) and ethanol (3 times, 300 mL each), filter and collect, and put it in an oven Dry at 80°C for 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com