Near-infrared fluorescent probe targeting cyp1b1 enzyme and its preparation and use
A fluorescent probe, near-infrared technology, used in fluorescence/phosphorescence, preparations for in vivo tests, luminescent materials, etc., can solve the problems of difficult diagnosis and low diagnostic specificity, achieve deep non-destructive testing, promote application, Effect with application prospect and clinical translation value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
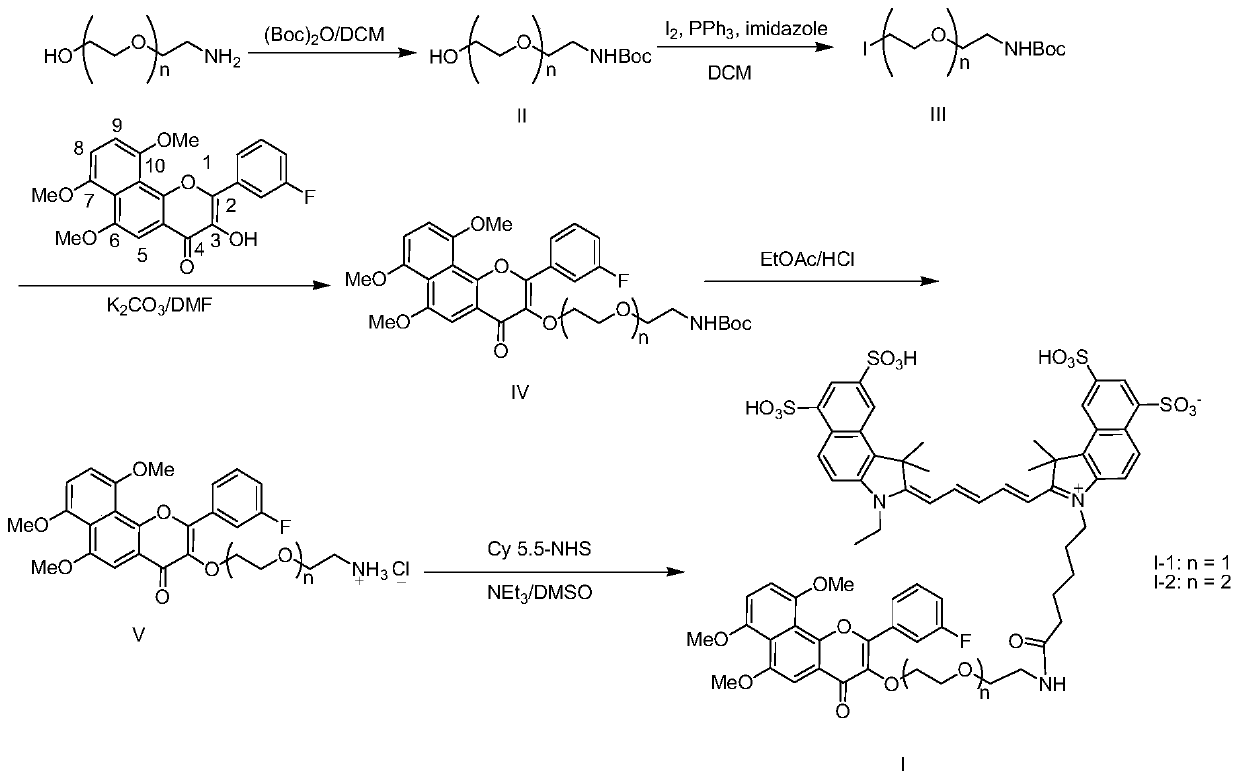
[0037] This embodiment relates to a preparation method of near-infrared fluorescent probe I-1 derived from 6,7,10-trimethoxy-3'-fluoro-α-naphthalene flavonol with structural formula I, such as figure 1 shown, including the following steps:
[0038] Step 1: 2-(2-Aminoethoxy)ethanol (2 mmol) was dissolved in 6 mL of dichloromethane, and di-tert-butyl dicarbonyl ester (2.3 mmol) dissolved in 4 mL of dichloromethane was added dropwise under ice-cooling. After the dropwise addition, the ice bath was removed, and the reaction solution was stirred overnight at room temperature. After the reaction, the reaction solution was diluted with 10 mL of dichloromethane, and the organic phase was washed successively with an equal volume of water, saturated sodium bicarbonate solution and saturated sodium chloride solution. After drying the organic phase with anhydrous sodium sulfate, it was concentrated under reduced pressure to obtain a colorless oily substance tert-butoxyacyl 2-(2-hydroxyet...
Embodiment 2
[0044] This embodiment relates to a preparation method of near-infrared fluorescent probe I-2 derived from 6,7,10-trimethoxy-3'-fluoro-α-naphthalene flavonol with structural formula I, such as figure 1 shown, including the following steps:
[0045] Step 1: Same as Step 1 of Example 1, with 2-(2-(2-aminoethoxy)ethoxy)ethanol instead of 2-(2-aminoethoxy)ethanol to obtain a colorless oily tert-butoxy Acyl 2-(2-(2-hydroxyethoxy)ethoxy)ethylamine II-2 (n=2), yield: 98%. 1 HNMR (400MHz, CDCl3 ):3.76(t, J=4.4Hz, 2H), 3.61-3.65(m, 6H), 3.56(t, J=5.2Hz, 2H), 3.32(t, J=5.2Hz, 2H), 1.45(s ,9H).
[0046] Step 2: Same as Step 2 of Example 1, substituting II-1 for II-1 to obtain colorless oily tert-butoxyacyl 2-(2-(2-iodoethoxy)ethoxy)ethylamine III -2 (n=2), yield: 70%. 1 HNMR (400MHz, CDCl 3 ):5.03(br,1H),3.76(t,J=6.8Hz,2H),3.64-3.66(m,4H),3.56(t,J=4.8Hz,2H),3.26-3.34(m,4H) ,1.45(s,9H).
[0047] Step 3: Same as step 3 of Example 1, substituting III-1 with III-2 to obtain 3'-fluoro-...
Embodiment 3
[0051] The inhibitory activity of the α-naphthoflavone derivatives V-1 and V-2 with PEG chains obtained in Examples 1 and 2 on CYP1A1, CYP1A2, and CYP1B1 enzymes was determined.
[0052] In this experiment, 7-ethoxy-3H-phenoxazine 3-keto-deethoxy (EROD) assay was used to determine its inhibitory activity and selectivity to CYP1A1, CYP1A2, and CYP1B1 enzymes (Yamaori et al, Biochem.Pharmacol.2010 ,79:1691-1698.) The reaction system (200 μL) contains CYP1A1 (10fmol), CYP1A2 (60fmol) or CYP1B1 (20fmol), different concentrations of the test compound, NADPH regeneration system (1.3mM NADPNa2, 3.3mM glucose-6-phosphate , 0.5U / ml glucose-6-phosphate-dehydrogenase), 3.3mM magnesium chloride solution and 150nM 7-ethoxy-3H-phenoxazin-3-one. Five replicate wells were set up for each experimental group or control group as a parallel experiment. The reaction buffer was 50 mM Tris-HCl (pH 7.4) buffer containing 1% BSA solution. After the reaction system was preheated at 37°C for 5 minutes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com