Synthesis method of nickel bisoxalate borate and application thereof
A bisoxalate boric acid and a synthesis method technology, applied in the field of electrochemical materials, can solve the problems of high price and poor performance, and achieve the effects of short reaction time, easy operation and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] S1, take by weighing 11.73 g oxalic acid, 2.88 g boric acid, 2.94 g nickel chloride;
[0024] S2. Heat and dry the weighed oxalic acid at 100°C for 3 h in vacuum, and heat and dry boric acid and nickel chloride at 65°C for 3 h without vacuum;
[0025] S3. Place the dried oxalic acid, boric acid, and nickel chloride in a round bottom flask, shake well (about 1 hour), press into tablets at 10 Mpa, transfer to a beaker, and place in a vacuum drying oven at 120 °C React for 30 min, vacuumize to 0.07 Mpa, and react for 12 h at a temperature of 150 °C to obtain a white solid;
[0026] S4, transfer the obtained white solid into 500 mL of acetonitrile, filter after stirring for 15 min, and evaporate the solvent of the obtained filtrate at 65°C to obtain about 6.8 g of nickel borate bisoxalate, with a conversion rate of about 68% and a purity of about 98.5%.
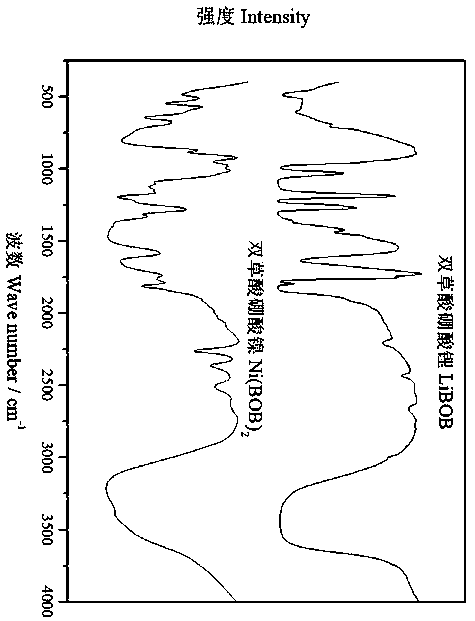
[0027] The infrared spectrogram of the nickel bisoxalate borate prepared by the present embodiment is shown in figure...
Embodiment 2
[0029] S1, take by weighing 19.33 g oxalic acid, 4.74 g boric acid, 1.99 g nickel chloride;
[0030] S2. Heat and dry the weighed oxalic acid at 110°C for 3 h in vacuum, and heat and dry boric acid and nickel chloride at 65°C for 3 h without vacuum;
[0031] S3. Put the dried oxalic acid, boric acid, and nickel chloride in a round bottom flask, shake well (about 1h), press into tablets at 10 Mpa, transfer to a beaker, react at 120°C for 45 minutes, and place in The vacuum was evacuated to 0.07 Mpa in a vacuum drying oven, and the temperature was set at 150 °C for 12 h to obtain a white solid.
[0032] S4. Transfer the obtained white solid to 500 mL of dimethyl carbonate, filter after stirring for 15 min, and evaporate the solvent from the obtained filtrate at 65°C to obtain about 9.48 g of nickel bisoxalate borate, and the primary conversion rate is about 63.2% , the purity is about 99%.
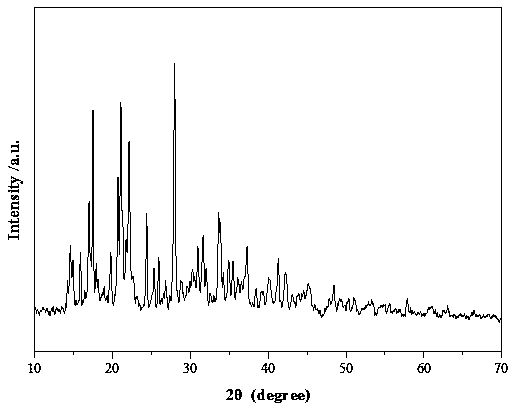
[0033] figure 2 The XRD spectrum of the nickel bisoxalate borate prepared in this ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com