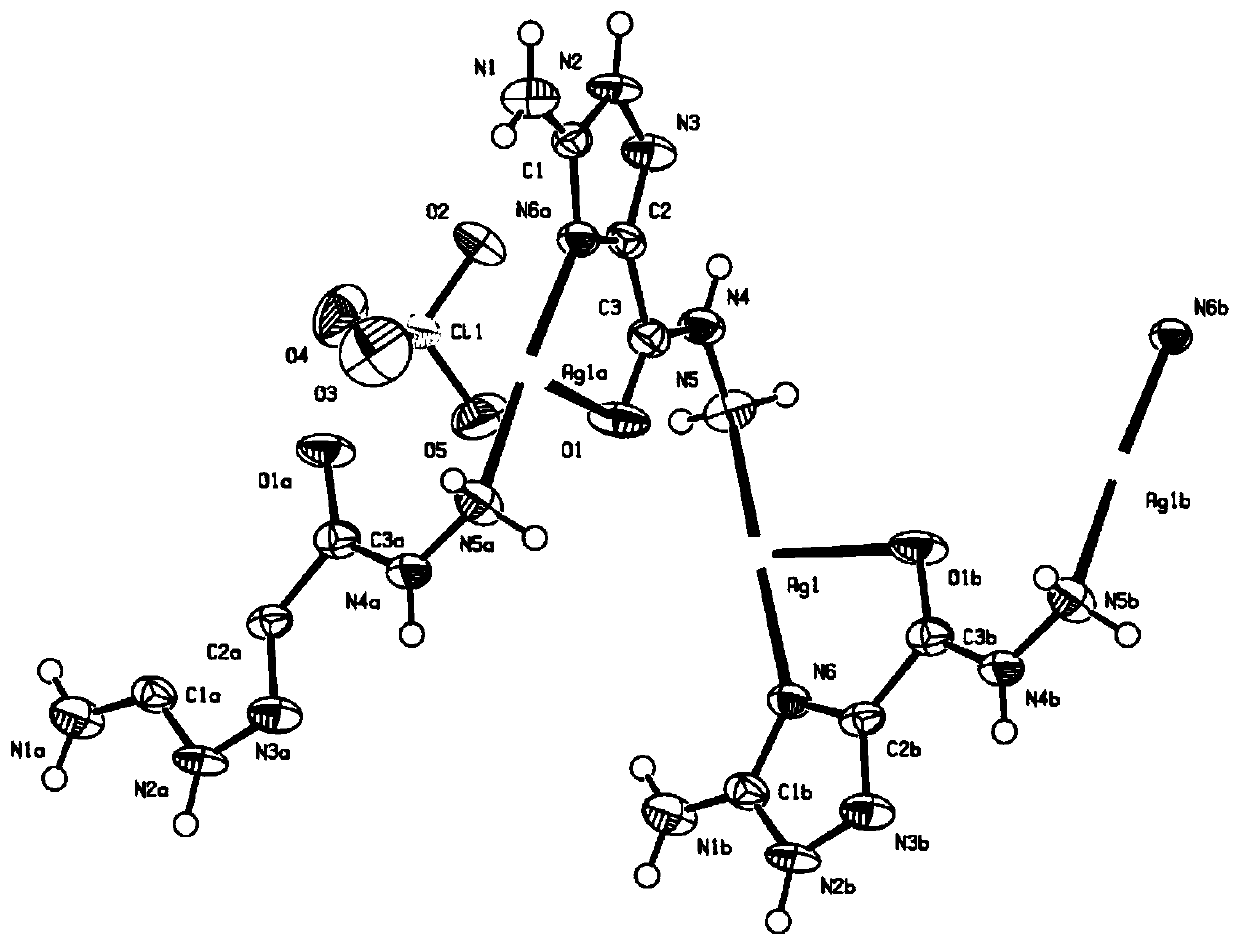
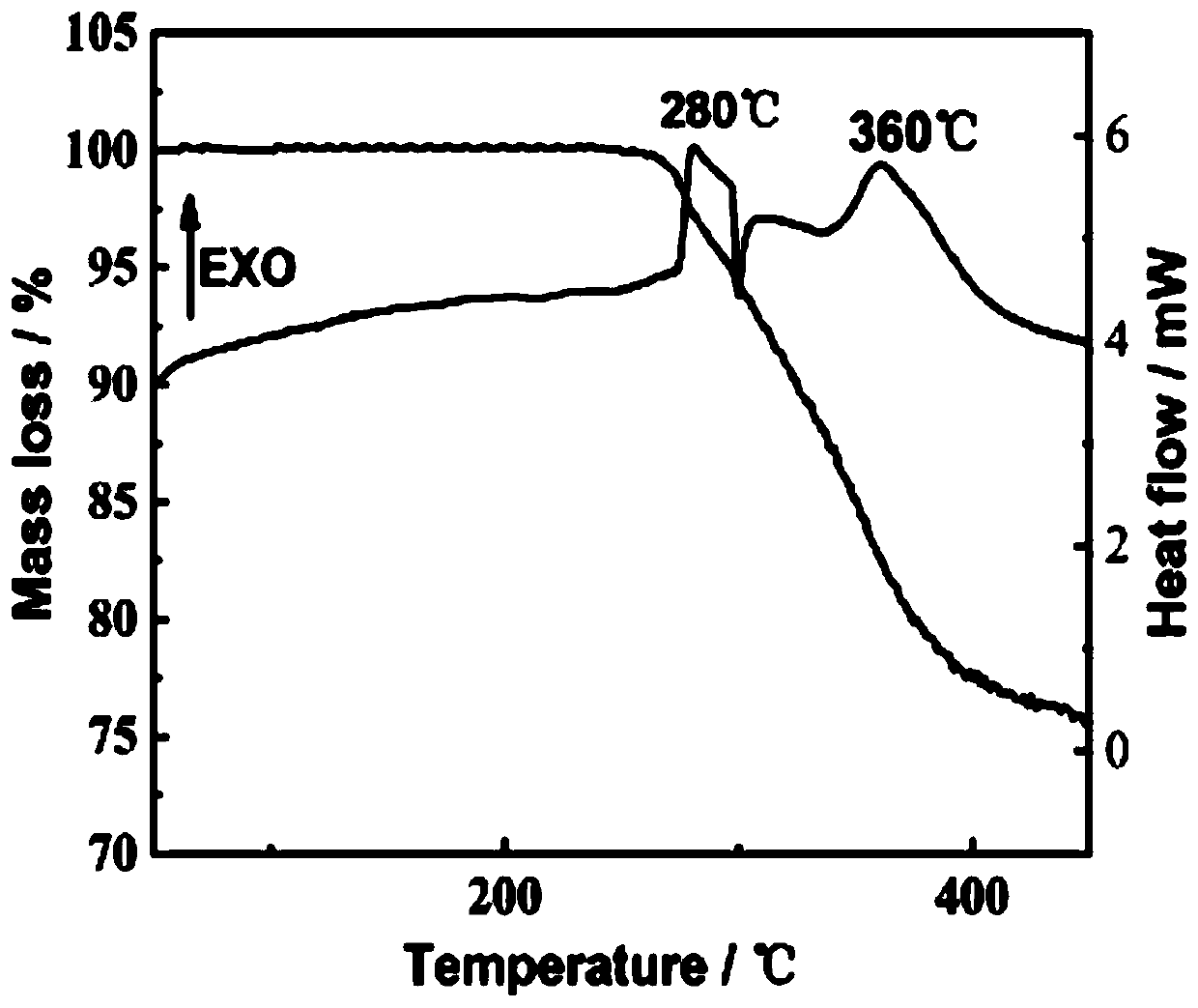
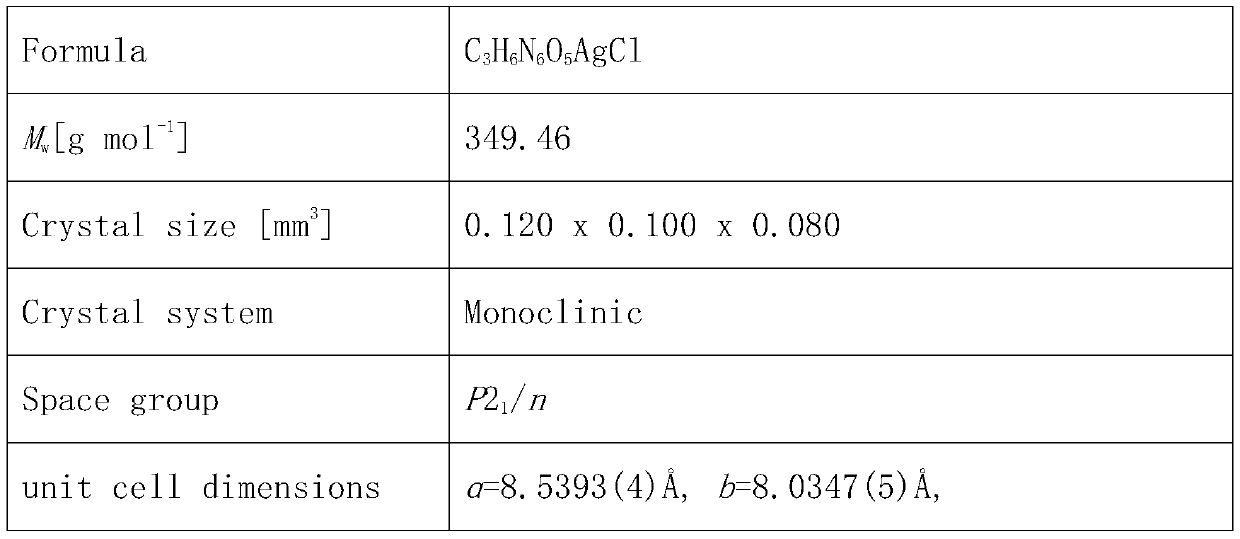
Insensitive energetic metal organic framework and preparation method thereof
A metal-organic framework, a certain amount of technology, applied in the direction of explosives, etc., can solve the problems of increasing the complexity of ECMOF, and achieve the effects of good safety and energetic performance, good safety, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Step 1: Synthesis of methyl 5-amino-1H-1,2,4-triazole-3-carboxylate (MATC)
[0029] Weigh 12.8g (10mmol) of 3-amino-1H-1,2,4-triazole-5-carboxylic acid and 90mL of anhydrous methanol in a 250mL three-necked bottle, and slowly add 6mL of 50% fuming h 2 SO 4 . The temperature was maintained at 85°C and refluxed for 12 hours. After the reaction was completed, the mixed solution was cooled to room temperature, and then a part of methanol was removed by rotary evaporation to obtain a precipitate. Add 20mL of ice water to dissolve the precipitate with a concentration of 5mol L -1 The pH of the mixed solution was adjusted to 5-6 with NaOH solution, and a white precipitate was obtained immediately. The white precipitate was filtered and washed 2 to 3 times with absolute ethanol. After drying, methyl 3-amino-1H-1,2,4-triazole-5-carboxylate was obtained (yield 73%).
[0030] Step 2: Synthesis of 3-amino-1H-1,2,4-triazole-5-carbohydrazide (ATCA)
[0031] Add 4.26g (30mmol)...
Embodiment 2
[0035]Step 1: Synthesis of methyl 5-amino-1H-1,2,4-triazole-3-carboxylate (MATC)
[0036] Weigh 12.8g (10mmol) of 3-amino-1H-1,2,4-triazole-5-carboxylic acid and 90mL of anhydrous methanol in a 250mL three-necked flask, and slowly add 6mL of 50% fuming H 2 SO 4 . The temperature was maintained at 85°C and refluxed for 12 hours. After the reaction was completed, the mixed solution was cooled to room temperature, and then a part of methanol was removed by rotary evaporation to obtain a precipitate. Add 20mL of ice water to dissolve the precipitate with a concentration of 5mol L -1 The pH of the mixed solution was adjusted to 5-6 with NaOH solution, and a white precipitate was obtained immediately. The white precipitate was filtered and washed 2 to 3 times with absolute ethanol. After drying, methyl 3-amino-1H-1,2,4-triazole-5-carboxylate was obtained (yield 73%).
[0037] Step 2: Synthesis of 3-amino-1H-1,2,4-triazole-5-carbohydrazide (ATCA)
[0038] Add 4.26g (30mmol) of...
Embodiment 3
[0042] Step 1: Synthesis of methyl 5-amino-1H-1,2,4-triazole-3-carboxylate (MATC)
[0043] Weigh 12.8g (10mmol) of 3-amino-1H-1,2,4-triazole-5-carboxylic acid and 90mL of anhydrous methanol in a 250mL three-necked flask, and slowly add 6mL of 50% fuming H 2 SO 4 . The temperature was maintained at 85°C and refluxed for 12 hours. After the reaction was completed, the mixed solution was cooled to room temperature, and then a part of methanol was removed by rotary evaporation to obtain a precipitate. Add 20mL of ice water to dissolve the precipitate with a concentration of 5mol L -1 The pH of the mixed solution was adjusted to 5-6 with NaOH solution, and a white precipitate was obtained immediately. The white precipitate was filtered and washed 2 to 3 times with absolute ethanol. After drying, methyl 3-amino-1H-1,2,4-triazole-5-carboxylate was obtained (yield 73%).
[0044] Step 2: Synthesis of 3-amino-1H-1,2,4-triazole-5-carbohydrazide (ATCA)
[0045] Add 4.26g (30mmol) o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
| Impact sensitivity | aaaaa | aaaaa |
| Friction sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com