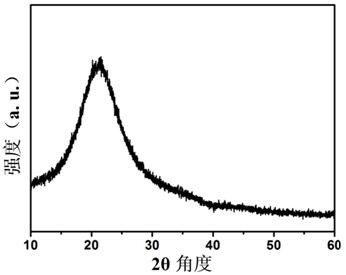
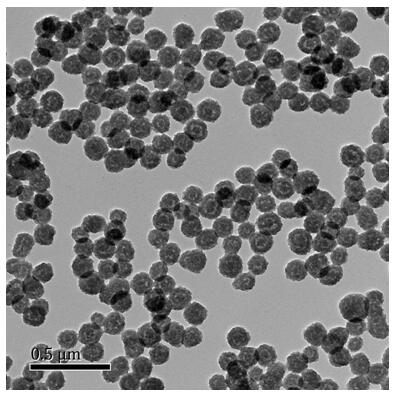
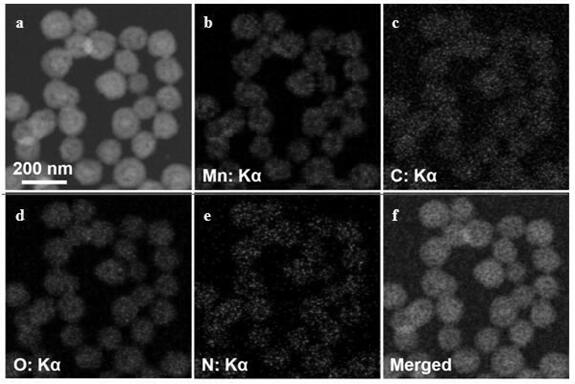
A kind of manganese carbonate/polyamino acid compound and preparation method thereof
A polyamino acid and compound technology, applied in the field of manganese carbonate/polyamino acid compound and its preparation, can solve the problems of large manganese carbonate size, low purity, difficulty in conforming to biomedical applications, etc., and achieve convenient operation and simple preparation process , Realize the effect of drug release monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) At room temperature, 1.980g MnCl 2 • 4H 2 O is dissolved in 10 mL of deionized water to form MnCl with a concentration of 1 mol / L 2 solution; 1.060g Na 2 CO 3 Dissolve in 100mL deionized water to form Na with a concentration of 0.1mol / L 2 CO 3 solution.
[0049] (2) Take 0.1mL, 1mol / L MnCl 2 The solution was added into 0.9mL Tris-HCl buffer (10mmol / L) and mixed evenly to obtain a manganese precursor solution; 0.1mL, 0.1mol / L Na 2 CO 3 The solution was mixed with 0.8 mL Tris-HCl buffer (10 mmol / L) to form a carbonate precursor solution.
[0050] (3) Take 0.1 mL of an aqueous solution containing 4 mg of polyethylene glycol-polyaspartic acid (the molecular weight of polyethylene glycol is 10,000, and the degree of polymerization of polyaspartic acid is 50) and mix it with the above-mentioned carbonate precursor solution , added to the above-mentioned manganese precursor solution, and stood in a refrigerator at 4°C for 12h.
[0051] (4) Then perform centrifuga...
Embodiment 2
[0053] (1) At room temperature, 1.980g MnCl 2 • 4H 2 O is dissolved in 10 mL of deionized water to form MnCl with a concentration of 1 mol / L 2 solution; 1.060g Na 2 CO 3 Dissolve in 100mL deionized water to form Na with a concentration of 0.1mol / L 2 CO 3 solution.
[0054] (2) Take 0.1mL, 1mol / L MnCl 2 The solution was added into 0.9mL Tris-HCl buffer (10mmol / L) and mixed evenly to obtain a manganese precursor solution; 0.1mL, 0.1mol / L Na 2 CO 3 The solution was mixed with 0.8 mL Tris-HCl buffer (10 mmol / L) to form a carbonate precursor solution.
[0055] (3) Take 0.1 mL of an aqueous solution containing 8 mg of polyethylene glycol-polyaspartic acid (the molecular weight of polyethylene glycol is 10,000, and the degree of polymerization of polyaspartic acid is 50) and mix it with the above-mentioned carbonate precursor solution , added to the above-mentioned manganese precursor solution, and stood in a refrigerator at 4°C for 12h.
[0056] (4) Then perform centrifuga...
Embodiment 3
[0058] (1) At room temperature, 1.980g MnCl 2 • 4H 2 O is dissolved in 10 mL of deionized water to form MnCl with a concentration of 1 mol / L 2 solution; 1.060g Na 2 CO 3 Dissolve in 100mL deionized water to form Na with a concentration of 0.1mol / L 2 CO 3 solution.
[0059] (2) Take 0.1mL, 1mol / L MnCl 2 The solution was added into 0.9mL Tris-HCl buffer (10mmol / L) and mixed evenly to obtain a manganese precursor solution; 0.1mL, 0.1mol / L Na 2 CO 3 The solution was mixed with 0.8 mL Tris-HCl buffer (10 mmol / L) to form a carbonate precursor solution.
[0060] (3) Take 0.1 mL of an aqueous solution containing 4 mg of polyethylene glycol-polyaspartic acid (the molecular weight of polyethylene glycol is 10,000, and the degree of polymerization of polyaspartic acid is 50) and mix it with the above-mentioned carbonate precursor solution , added to the above-mentioned manganese precursor solution, and stood in a 4°C refrigerator for 1h.
[0061] (4) Then perform centrifugation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com