Alpha-hydroxy pinacol borate compound preparation method
A technology of alcohol borates and compounds, which is applied in the field of preparation of α-hydroxy pinacol borate compounds, can solve the problems of difficult catalyst synthesis, high synthesis cost, sensitive steric hindrance, etc., and achieve good functional group compatibility and reaction High efficiency and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0012] One aspect of the embodiments of the present invention provides a method for preparing an α-hydroxy pinacol borate compound, comprising:
[0013] In a protective atmosphere, react the mixed reaction system comprising alcohol compound, double boron reagent, iron catalyst, ruthenium catalyst, phosphorus ligand, basic substance, hydrogen acceptor and solvent at 60-120°C for 1-10h, Then, a quenching agent is added to quench the reaction, and the α-hydroxy pinacol borate compound is obtained through post-treatment.
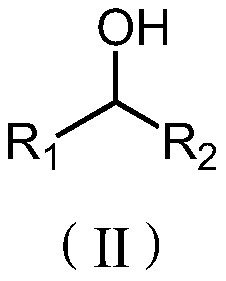
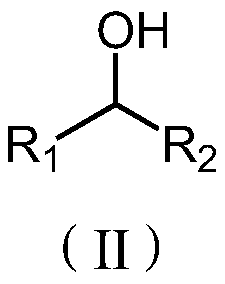
[0014] In some embodiments, the alcohol compound has the structure shown in formula (II):
[0015]
[0016] Among them, R 1 , R 2 selected from alkyl groups with 1 to 10 carbon atoms.
[0017] Further, the molar ratio of alcohol compound, double boron reagent, iron catalyst, ruthenium catalyst, phosphorus ligand, basic substance and hydrogen acceptor in the mixed reaction system is 1.0:(1.0~3.0):(0.02~0.5 ):(0.01~0.1):(0.01~0.1):(0.1~2.0):(0.1~2.0).
[0...
Embodiment 1
[0038] In the glove box, add biboronic acid pinacol borate, iron catalyst, ruthenium catalyst, ligand, base, hydrogen acceptor, toluene to the Schleck reaction tube, remove from the glove box, and add alcohol under nitrogen atmosphere The compound was then placed in the reaction tube at 100°C for 6 hours. Then adding a quenching agent to quench the reaction, adding ethyl acetate to the system for extraction, and separating by column chromatography to obtain the product.
Embodiment 2-20
[0040] In the glove box, add biboronic acid pinacol borate, iron catalyst, ruthenium catalyst, ligand, base, hydrogen acceptor, toluene to the Schleck reaction tube, remove from the glove box, and add alcohol under nitrogen atmosphere The compound is then placed in the reaction tube at 100°C for 1-6 hours. Then adding a quenching agent to quench the reaction, adding ethyl acetate to the system for extraction, and separating by column chromatography to obtain the product. Wherein, the molar ratio of alcohol compound, diboron reagent, iron catalyst, ruthenium catalyst, phosphorus ligand, basic substance and hydrogen acceptor is 1.0:1.7:0.1:0.02:0.02:0.4:1.0.
[0041] The structure and the productive rate of the alcohol compound used in the embodiment 1-21 of table 1
[0042]
[0043] The product that above all embodiment 1-20 obtains all passes through 1 H-NMR, 13 C-NMR characterization was confirmed, and all unknown samples were confirmed by high-resolution mass spectrome...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com