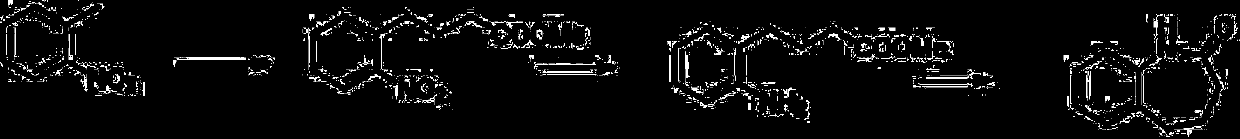Synthesis method of benzocaprolactam
A technology of benzocaprolactam and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of dangerous materials, use, and high cost, and achieve the effects of low cost, simple post-processing operation, and high output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A kind of synthetic method of benzocaprolactam of the present invention comprises the following steps:
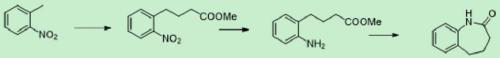
[0030] S1, using o-nitrotoluene as raw material, condensing reaction with acrylate under alkali catalysis to obtain o-nitrobenzene butyric acid or its ester;
[0031] S2. O-nitrophenylbutyric acid or its ester reduces the nitro group and closes the ring to obtain the target product. The reaction principle of the present invention is as figure 1 shown.
[0032] The concrete implementation mode of the present invention is:
[0033] Preparation of methyl o-nitrobenzenebutyrate: put 500 g of o-nitrotoluene, 20 g of methyl acrylate, and 1 g of a phase transfer catalyst (such as tetrabutylammonium bromide) into a four-necked flask; heat up to 35° C., and slowly add hydrogen dropwise Concentrated potassium hydroxide aqueous solution prepared with 13 g of potassium oxide; react for 1 hour after the dropwise addition, add 200 g of water, and separate layers to obtain an aq...
Embodiment 2
[0037] The concrete implementation mode of the present invention is:
[0038] Preparation of methyl o-nitrobenzenebutyrate: put 3000 g of o-nitrotoluene, 200 g of methyl acrylate, and 10 g of a phase transfer catalyst (such as tetrabutylammonium bromide) into a four-necked flask; heat up to 35 ° C, slowly dropwise add hydrogen to oxidize Concentrated potassium hydroxide aqueous solution prepared with 130g of potassium; after the dropwise addition, react for 1 hour, add dilute sulfuric acid to adjust the pH to 1; add 1000g of dichloromethane for extraction three times; combine dichloromethane, dry over sodium sulfate, concentrate to remove solvent, and vacuum distill In addition to o-nitrotoluene, the black oil obtained was methyl o-nitrobenzene butyrate, with a yield of 77% and a purity of 68% by HPLC; 1H NMR (400MHz, DMSO) δ1.90-1.97 (m, 2H), 2.32– 2.36(m,2H),2.84–2.88(m,2H),3.61(s,3H),7.29–7.32(m,2H),7.45–7.49(m,1H),7.81–7.83(d,1H);
[0039] Preparation of benzocaprolactam:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com