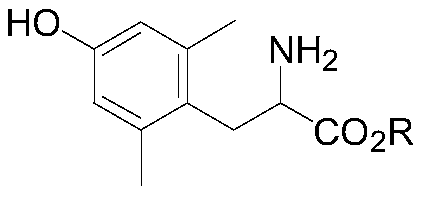
Resolution method for 2,6-dimethyltyrosine ester and application thereof
A technology of dimethyl tyrosine ester and tyrosine ester, applied in the direction of organic chemical methods, chemical instruments and methods, separation of optically active compounds, etc., can solve problems such as harsh conditions, high production costs, and expensive prices, and achieve Improve the resolution yield, convenient operation, and facilitate the effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Dissolve 245g of racemic 2,6-dimethyl-tyrosine in 1L of ethanol, drop into 179g of thionyl chloride at low temperature, heat to reflux for 2 hours, and concentrate to dryness after the reaction. Add water and dichloromethane for extraction, adjust the pH to 9-10 with 10% aqueous sodium hydroxide solution, separate layers, extract the aqueous phase once more, combine the organic phases and wash with water, and concentrate the organic phases to dryness to obtain about 225 g of 2,6 -Dimethyl-tyrosine ethyl ester.
Embodiment 2
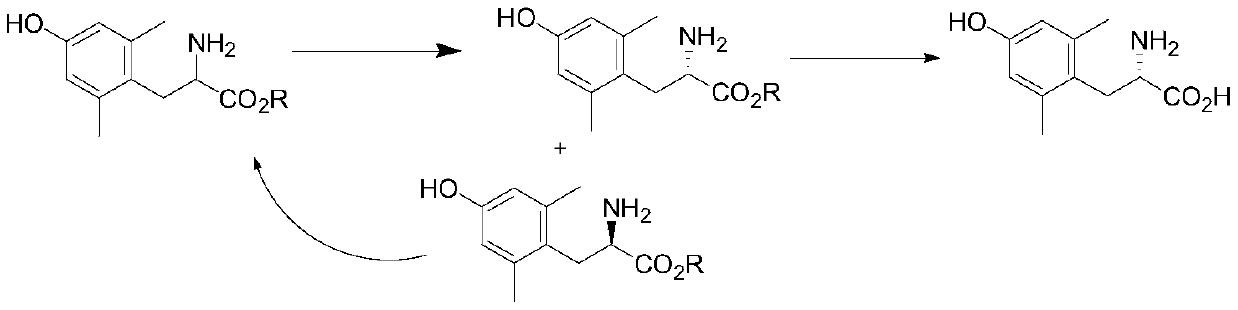
[0040] Dissolve 238g of L-dibenzoyltartaric acid in 800g of isopropanol, heat to 70 degrees, slowly drop into 300g of isopropanol solution of 100g of 2,6-dimethyl-tyrosine ethyl ester, add a small amount of seed crystals , more crystals were precipitated, continued to stir at 70°C for 2 hours, cooled to 30°C, kept warm for 1 hour, filtered with suction, and the solid was washed with isopropanol at room temperature. The mother liquor is subjected to the next racemization operation, and the filter cake is blown and dried at 50 degrees to obtain the L-dibenzoyl tartrate product of 2,6-dimethyl-L-tyrosine ethyl ester. The yield is 38%, and the ee value is 96%.
Embodiment 3
[0042] Add dichloromethane and water to the mother liquor obtained in the previous step, adjust the pH to 9-10 with 10% aqueous sodium hydroxide solution, separate layers, extract the water phase once more, combine the organic phases and wash with water, and concentrate the organic phases to dryness. Add 1g of concentrated sulfuric acid, 5g of salicylaldehyde, and 300g of isopropanol, and reflux for 4 hours. Concentration to dryness recovered racemic 2,6-dimethyl-tyrosine ethyl ester. The recovery yield was 70%. Simultaneously, the aqueous phase also recovers L-dibenzoyl tartaric acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com