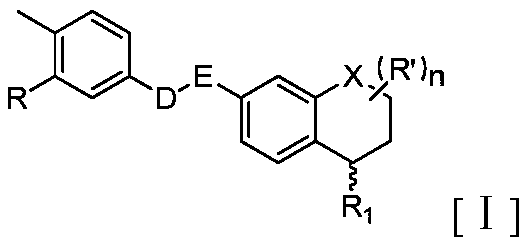
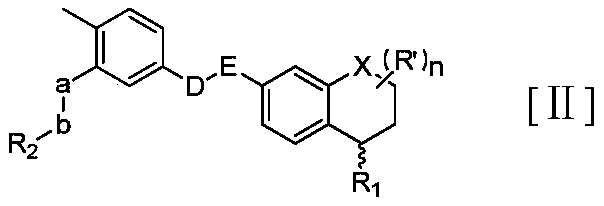
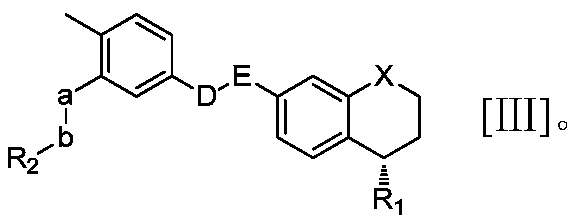
Novel S-type or R-type tetrahydronaphthalene amide compounds and pharmaceutically acceptable salts thereof, and preparation method and application thereof
A technology of tetralinamide and compound, applied in the field of medicine, can solve problems such as death, adverse reactions of patients, steric hindrance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1: (S)-N-(3-(imidazo[1,2-b]pyridazin-3-ethynyl)-4-methylphenyl)-5-(4-methylpiperazine) Preparation of -5,6,7,8-tetrahydronaphthalene-2-carboxamide (compound 1)
[0109]
[0110] Step A: Synthesis of (S)-methyl 5-hydroxy-5,6,7,8-tetralin-2-carboxylate
[0111]
[0112] Put 10 ml of 1.0 M (R)-2-methyl-CBS-oxazoboridine (0.1 mmol) in a round-necked flask, add toluene to dissolve, lower to -10°C, add 25 ml of borane dimethyl Toluene solution of the complex compound of thioether (2 mmol) was slowly added dropwise with 40 ml of methyl 5-carbonyl-5,6,7,8-tetrahydronaphthalene-2-carboxylate (5 g , 1 mmol), the dropwise addition was completed within half an hour, stirred for 1 h, added 20 ml of methanol and continued to stir at 0°C for 2 h. Rotary evaporation removed methanol, then extracted with 100 ml of ethyl acetate, the organic phase was washed with 1M phosphoric acid solution, water, saturated saline solution, dried over anhydrous sodium sulfate, filtered, ...
Embodiment 1-1
[0134] Example 1-1: (S)-N-(3-(imidazo[1,2-b]pyridazin-3-ethynyl)-4-methylphenyl)-5-(4-methylpiper Preparation of oxazin-1-yl)-5,6,7,8-tetralin-2-carboxamide hydrochloride (compound 1 hydrochloride)
[0135]
[0136] Add (S)-N-(3-(imidazo[1,2-b]pyridazin-3-ethynyl)-4-methylphenyl)-5-( 4-methylpiperazin-1-yl)-5,6,7,8-tetrahydronaphthalene-2-carboxamide (506 mg, 1 mmol), hydrochloric acid (202 mg, 1.05 mmol), absolute ethanol 15 ml, warmed up to 80°C and refluxed and stirred for half an hour, then cooled naturally to room temperature, a solid precipitated, filtered, washed with 5 ml of cold ethanol, filtered, and recrystallized with absolute ethanol to obtain 558 mg of a white solid product, the yield 80%.
Embodiment 2
[0137] Example 2: (R)-N-(3-(imidazo[1,2-b]pyridazin-3-ethynyl)-4-methylphenyl)-5-(4-methylpiperazine- Preparation of 1-yl)-5,6,7,8-tetrahydronaphthalene-2-carboxamide (compound 2):
[0138]
[0139] Step A: Synthesis of (R)-methyl 5-hydroxy-5,6,7,8-tetralin-2-carboxylate
[0140]
[0141] Referring to the method of embodiment 1 step A. Simply substituting (R)-2-methyl-CBS-oxazoborin (0.1 mmol) for (S)-2-methyl-CBS-oxazaborin (0.1 mol) afforded product 4.1 g, yielding The rate is 88.2%, and the ee value detected by chiral OD-H column is 98%. MS: m / z, 207.2 ([M+H] + )
[0142] Step B: Synthesis of (R)-methyl 5-chloro-5,6,7,8-tetralin-2-carboxylate
[0143]
[0144] Referring to the method of embodiment 1 step B. Just replace (S)-methyl 5-hydroxy-5,6,7,8-tetralin-2-carboxylate with (R)-5-hydroxy-5,6,7,8-tetralin- Methyl 2-carboxylate, 3.08 g of product was obtained, yield 92.6%. MS: m / z, 225.2 ([M+H] + )
[0145] Step C: Synthesis of (R)-methyl 5-(4-methylpiper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com