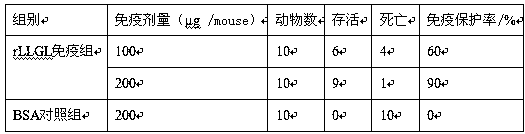
Preparation method of leptospira interrogans MAP vaccine
A leptospira and vaccine technology, applied in the field of cellular immunity, can solve the problems of high cost, single antigen, poor immune effect, etc., and achieve the effect of good antigenicity and cross-immunity reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
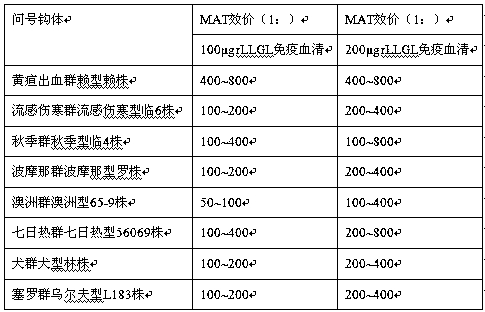
[0015] Example 1: Epitope prediction and identification
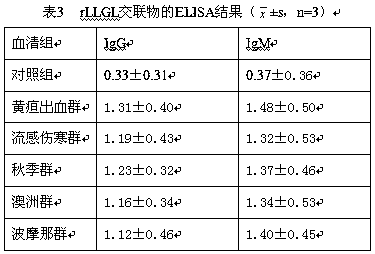
[0016] According to the protein primary structure sequence obtained from the lipL21, lipL32, groEL and loa22 gene sequences of the reference standard strains of Leptospira serotypes in my country, T cells were predicted by using the Propred HLA-DR binding peptide prediction-ProPred server and the ANTIGENIC program in the EMBOSS software package epitopes and B cell epitopes. The predicted epitope sequence was cloned into the phage vector M13KE to display it on the surface of PⅢ protein, and the display product was purified and analyzed for immune activity. The previous research results showed that the epitope composed of amino acids 97-122 and 176-184 of LipL21 protein, LipL32 The epitope composed of amino acids 133-160 and 221-247 of the protein, the epitope composed of amino acids 215-247 of the GroEL protein, and the epitope composed of amino acids 22-90 of the Loa22 protein have strong immune activity. The epitope seq...
Embodiment 2
[0020] Embodiment 2: Construction of gene synthesis and expression vector
[0021] The nucleotide sequence corresponding to each epitope is determined according to the amino acid sequence of the LLGL multivalent epitope and constituent epitope and the preferred codon characteristics of the expression host Escherichia coli, and the flexible peptide linker GGGGS nucleotide sequence ggtggcggtggcagc is used to connect each epitope, and in LLGL The 5' end of the polyvalent epitope coding sequence is the restriction endonuclease site EcoR I and the start codon ATG, and the 3' end is the restriction endonuclease site BglII, the stop codon TAA and BamH I in turn, entrusted Synthesized by Shanghai Invitrogen Company. The nucleotide sequence of the LLGL polyvalent epitope gene is shown below. The synthetic gene fragment was digested and purified by BamH I and EcoR I, then subcloned into E. coli expression vector pET-28a, transformed into E. coli BL21, and constructed a prokaryotic expr...
Embodiment 3
[0024] Embodiment 3: Expression and purification of recombinant protein
[0025] to pick E. coli BL21 pET-28a-LLGL A single colony was inoculated into 10 ml of Kana-resistant LB medium, and cultured overnight at 37°C with shaking. Transfer the culture medium to 10 ml of fresh LB medium resistant to kanamycin at a ratio of 1:100, culture with shaking at 37°C for 3 h, add IPTG to make the final concentration 1 mmol / L, and culture with shaking at 37°C 4 h. Take 1.5 ml of the culture solution and collect the cells by centrifugation, then resuspend them in 150 µl of PBS (pH7.4) buffer solution and perform low-temperature ultrasonication (300 V, 10S x5) to disrupt the cells. After centrifugation at 4°C and 12 000 r / min for 5 min, the supernatant and precipitate were collected for SDS-PAGE analysis. Re-inoculate the high-expression strains into 500 ml LB medium resistant to kanamycin to induce the expression of rLLGL in large quantities. After sonication, the recombinant protei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com