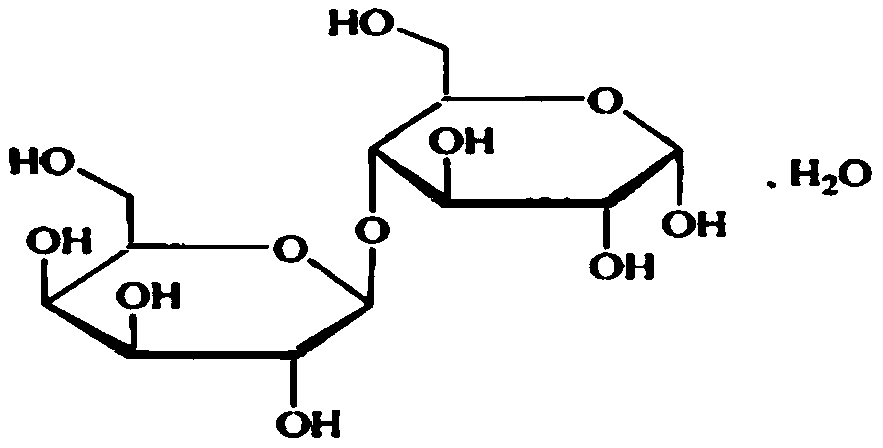
Lactose for injection and preparation method thereof
A technology of lactose for injection and medicinal lactose, which is applied in the field of medicine, can solve problems such as urticaria, cough, fever, shock, failure to meet the quality standards of injection-grade lactose, and restrictions on the application of lactose, so as to solve adverse reactions and improve freezing. Dry protection efficiency, simple and feasible purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
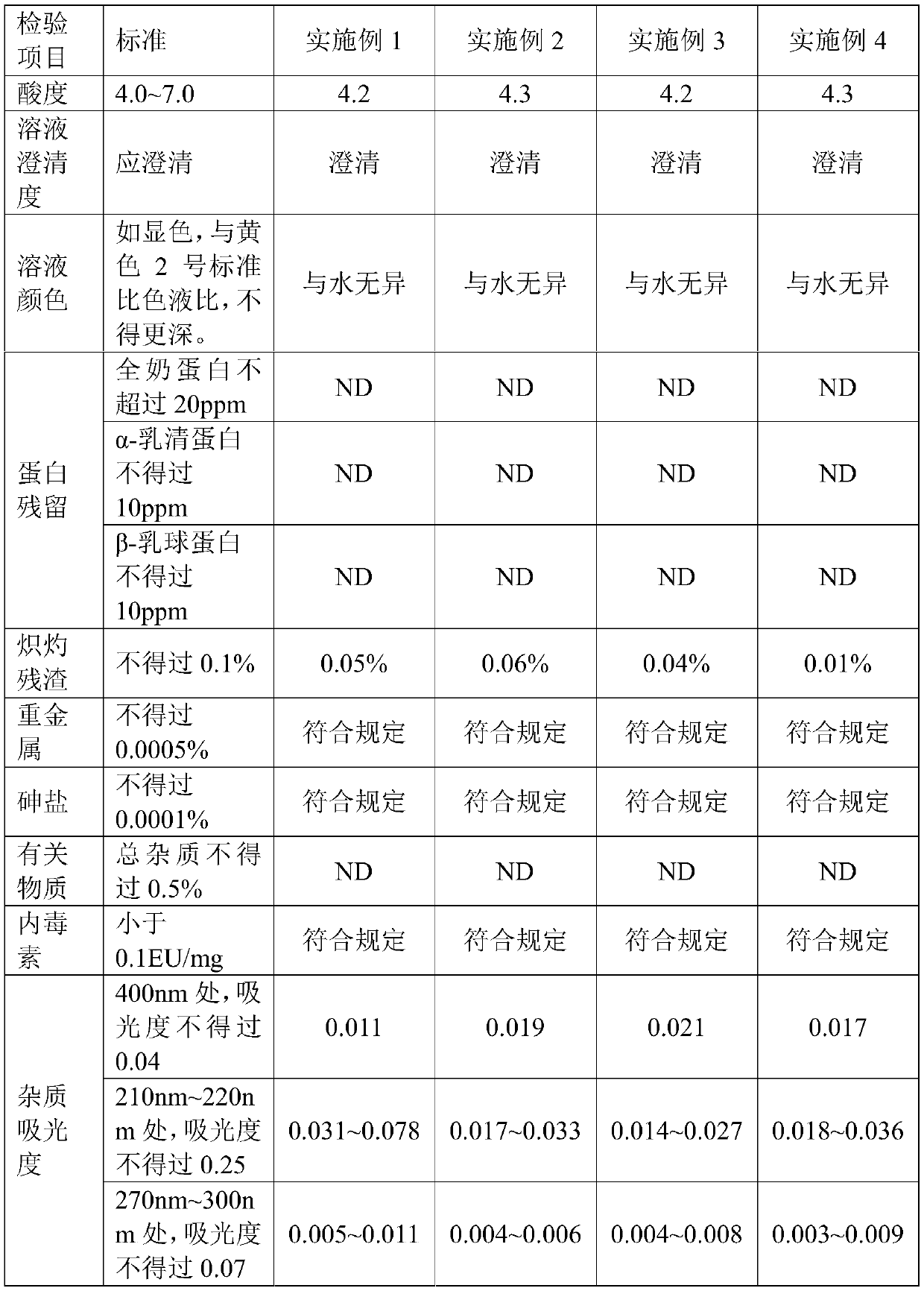
Embodiment 1
[0022] (1) Weigh 100g of commercially available common lactose monohydrate or medicinal lactose, dissolve in purified water and set the volume to 1000mL, heat to 50°C to dissolve for 100min, and use 10KD membrane bag for ultrafiltration after cooling down to room temperature;
[0023] (2) Concentrated crystallization: Concentrate the ultra-filtered lactose solution at 80°C, heat and stir the concentrated solution at 18°C for 13 hours, then stand at 8°C for 1 hour to crystallize;
[0024] (3) Drying: filter the lactose solution after static crystallization, and then vacuum dry at 90°C for 10 hours;
[0025] (4) Pulverization: After drying, the lactose is pulverized to obtain the product; the yield is 78.5%.
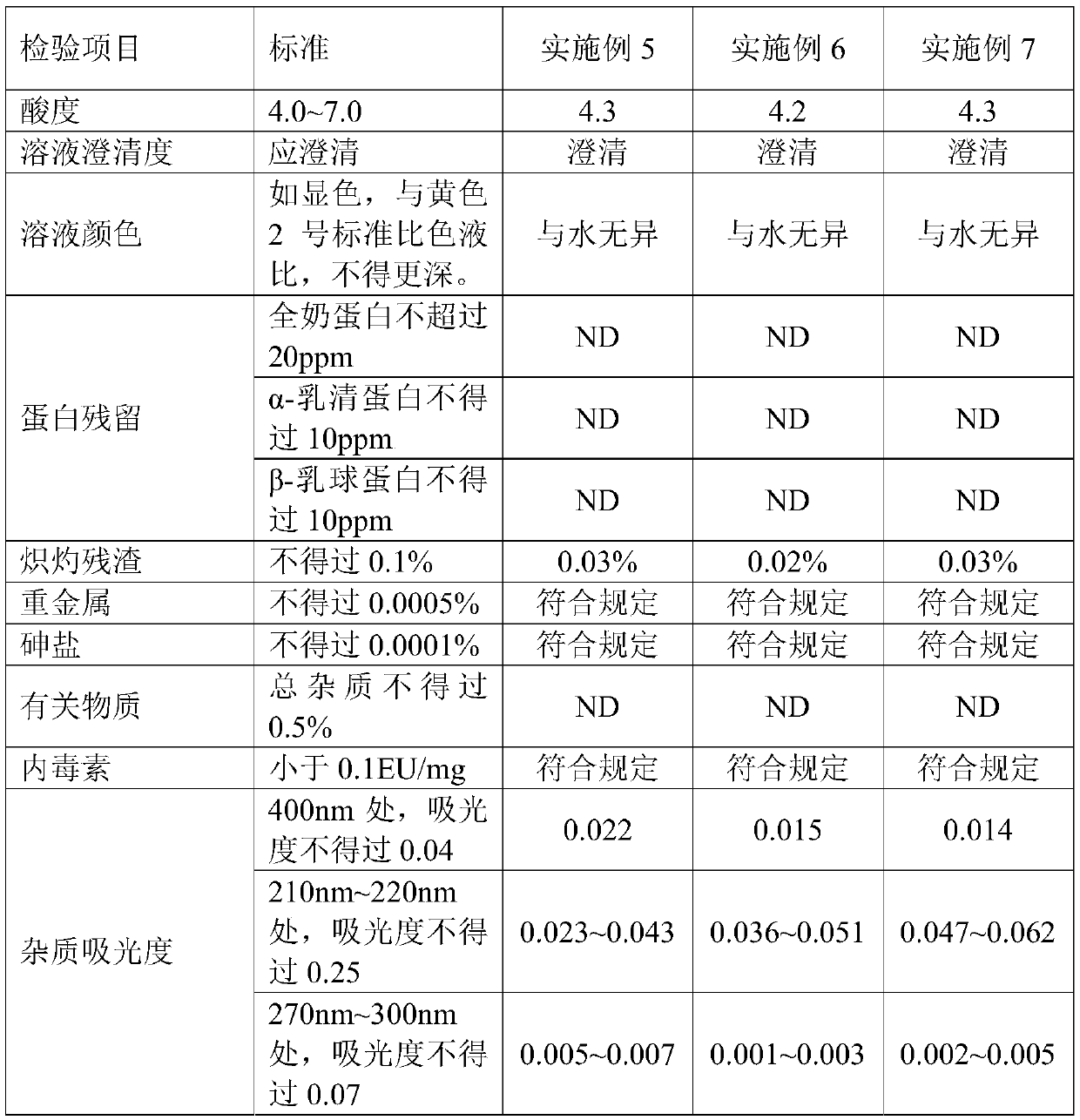
Embodiment 2
[0027] (1) Weigh 5Kg of commercially available common lactose monohydrate or medicinal lactose, dissolve in purified water and set the volume to 16.2L, heat to 90°C to dissolve for 20min, and use a 30KD membrane bag for ultrafiltration after cooling down to room temperature;
[0028] (2) Concentrated crystallization: Concentrate the ultra-filtered lactose solution at 70°C, heat and stir the concentrated solution at 28°C for 18 hours, then stand at 5°C for 6 hours;
[0029] (3) Drying: filter the lactose solution after static crystallization, and then vacuum dry at 70°C for 32 hours;
[0030] (4) Pulverization: After drying, the lactose is pulverized to obtain the product; the yield is 75.3%.
Embodiment 3
[0032] (1) Weigh 10Kg of commercially available common lactose monohydrate or medicinal lactose, dissolve it in purified water and set the volume to 24.4L, heat to 80°C to dissolve for 60min, and use 3KD membrane bag for ultrafiltration after cooling down to room temperature;
[0033] (2) Concentrated crystallization: Concentrate the ultra-filtered lactose solution at 80°C, heat and stir the concentrated solution at 20°C for 26 hours, and then stand at 2°C for 8 hours;
[0034] (3) Drying: filter the lactose solution after static crystallization, and then vacuum dry at 80°C for 24 hours;
[0035] (4) Pulverization: After drying, the lactose was pulverized to obtain the product; the yield was 82.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com