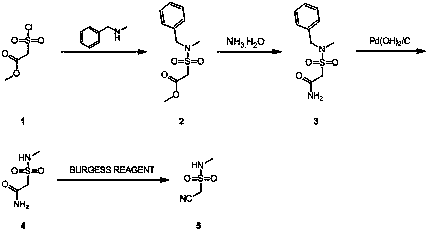
Synthesis method of 1-cyano-N-methylmethanesulfonamide
The technology of a kind of methyl methanesulfonamide and synthesis method is applied in the field of practical synthesis of 1-cyano-N-methyl methanesulfonamide, which can solve the problem that 2-(chlorosulfonyl) methyl acetate is expensive and cannot be realized. Industrialization and other issues, to achieve the effect of reasonable reaction process design and cost-saving synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
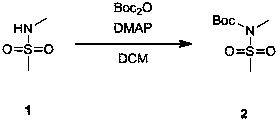
[0012] Synthesis of N-tert-butoxycarbonyl-methanesulfonamide (Compound 2)
[0013]
[0014] Add 300 g of compound 1 in dichloromethane (1500 mL) into a 3000 mL three-necked flask, and cool down to 0-5 °C. Add 629g of Boc anhydride and 17g of 4-N,N lutidine to the reaction system at low temperature (0-5°C), and at room temperature (15-30 o C) Stirring for 12-16 hours. After the reaction, the solvent was evaporated by rotary evaporation and purified by column chromatography to obtain 550 g of compound 2 as a colorless oil. HNMR(400MHz, CHLOROFORM-d) δ = 3.24 (s, 3H), 3.18 (s, 3H), 1.52 (s, 10H).
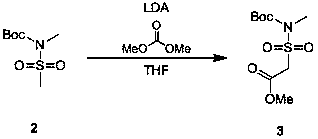
[0015] Synthesis of 1-methylformate-N-tert-butoxycarbonyl-methylmethanesulfonamide (compound 3)
[0016]
[0017] Add 430mL lithium diisopropylamide tetrahydrofuran solution dropwise into 150g compound 2 tetrahydrofuran solution (1500mL), stir at -65℃~-78℃ for 1 hour, then add 66g dimethyl carbonate into the system dropwise, -65℃ After reacting for 2 hours at ~-78°C, add 1500 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com