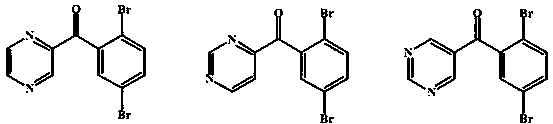
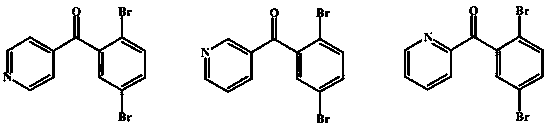
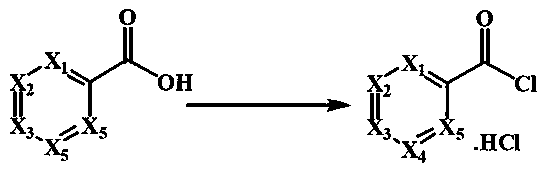
Preparation method of 4-(2,5-dibromobenzoyl) aza-aromatic ring
A technology for dibromobenzoyl and azaaromatic rings, which is applied in the field of preparation of 4-azaaromatic rings, can solve problems such as difficult bromination reactions and decreased reactivity of benzene rings, and achieve stable and feasible processes, good quality, The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of the hydrochloride of embodiment 1 4-pyridinecarbonyl chloride
[0025] Dissolve 12.3 grams of 4-pyridinecarboxylic acid in 50 mL of dioxane, add 14.5 mL of thionyl chloride, add 5 drops of N, N-dimethylformamide, heat to 105 ° C, react for four hours, evaporate the solvent and di Thionyl chloride was obtained as off-white solid, cooled, filtered, the filter cake was washed with petroleum ether, the solid was collected, dried under reduced pressure to obtain 16.8 g of product, yield: 94.9%.
Embodiment 2
[0026] Example 2 Preparation of 4-(2,5-dibromobenzoyl)pyridine
[0027] Under ice-bath conditions, 17.7 grams of 4-pyridineformyl chloride hydrochloride prepared according to Example 1, 26.6 grams of aluminum trichloride, and 58.5 grams of 1,4-dibromobenzene were mixed and stirred for 30 minutes, and the temperature was raised to 100 ° C. The reaction was completed in 2 hours. Pour the reactant into ice water, stir to decompose the complex, extract three times with dichloromethane, combine the organic layers, wash with water, wash with saturated sodium chloride, and dry over anhydrous magnesium sulfate. After filtration, the filtrate was concentrated under reduced pressure, and the residue was brownish yellow. After cooling, crystals were formed, and recrystallized with a mixed solvent of ethyl acetate and petroleum ether (2:1) to obtain light yellow transparent crystals, which were dried under reduced pressure to obtain 25.3 g of the product, yield: 75.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com