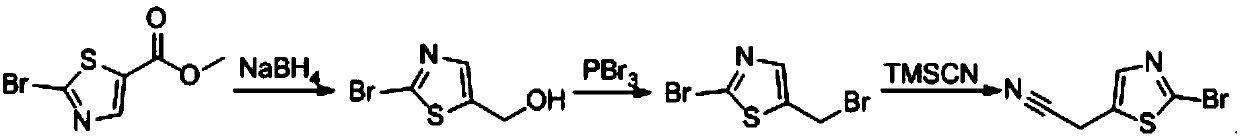
Preparation method of 2-(2-bromo-1,3-thiazol-5-yl) acetonitrile
A technology of bromothiazole and thiazole, which is applied in the field of preparation of 2-acetonitrile, achieves the effects of mild reaction conditions, safe and simple preparation process and purification steps, and convenient industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1), preparation of 2-bromothiazole-5-methanol:
[0024] Add methanol (50mL) 2-bromothiazole-5-methyl carboxylate (8.88g, 40mmol, 1.0eq) into a 100mL three-pin bottle, cool to 0°C, add sodium borohydride (2.88g, 80mmol, 2.0eq ), after the system was mixed uniformly, reacted at room temperature for 2h, TLC tracked the end of the reaction, concentrated the reaction solution, added 50mL water, and extracted three times with ethyl acetate (50mL X 3), combined the organic phases, dried over anhydrous sodium sulfate, concentrated, column Chromatographic separation gave 3.52 g of 2-bromothiazole-5-methanol, with a yield of 45%.
[0025] (2), utilizing the 2-bromothiazole-5-methanol obtained in step (1) to prepare 2-bromo-5-bromomethyl-thiazole:
[0026] Add dichloromethane (50mL), 2-bromothiazole-5-carboxylic acid methanol (3.88g, 20mmol, 1.0eq) in sequence in a 100mL three-pin bottle, cool to 0°C, add phosphorus tribromide (16.2g, 60mmol, 3.0eq), after the system was mixed ...
Embodiment 2
[0031] (1), preparation of 2-bromothiazole-5-methanol:
[0032] Add tetrahydrofuran (30mL), methyl 2-bromothiazole-5-carboxylate (4.4g, 20mmol, 1.0eq) into a 100mL three-pin bottle, cool to 0°C, and add sodium borohydride (1.4g, 40mmol, 2.0 eq), after the system was uniformly mixed, reacted at room temperature for 5 hours, TLC tracked the end of the reaction, concentrated the reaction solution, added 50 mL of water and extracted three times with ethyl acetate (50 mL × 3), combined the organic phases, dried over anhydrous sodium sulfate, concentrated, Column chromatography separated to obtain 3.1 g of 2-bromothiazole-5-methanol with a yield of 40%.
[0033] (2), utilizing the 2-bromothiazole-5-methanol obtained in step (1) to prepare 2-bromo-5-bromomethyl-thiazole:
[0034] Add dichloromethane (50mL), 2-bromothiazole-5-carboxylic acid methanol (3.88g, 20mmol, 1.0eq) in sequence in a 100mL three-pin bottle, cool to 0°C, add phosphorus tribromide (16.2g, 60mmol, 3.0eq), after t...
Embodiment 3
[0039] (1), preparation of 2-bromothiazole-5-methanol:
[0040] Add methanol / H 2 O (1:1, 50mL), methyl 2-bromothiazole-5-carboxylate (8.88g, 40mmol, 1.0eq), cooled to 0°C, added sodium borohydride (2.88g, 80mmol, 2.0eq) in portions, After the system was uniformly mixed, reacted at room temperature for 2 hours, TLC followed the completion of the reaction, concentrated the reaction solution, added 100 mL of water and extracted three times with ethyl acetate (50 mL×3), combined the organic phases, dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography 3.4 g of 2-bromothiazole-5-methanol was obtained with a yield of 43%.
[0041] (2), utilizing the 2-bromothiazole-5-methanol obtained in step (1) to prepare 2-bromo-5-bromomethyl-thiazole:
[0042]Add tetrahydrofuran (50mL), 2-bromothiazole-5-carboxylic acid methanol (3.88g, 20mmol, 1.0eq) successively into a 100mL three-pin bottle, cool to 0°C, add phosphorus oxybromide (17.2g, 60mmol, 3.0eq ),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com