Pseudo-polymorph of Arbekacin hydrochloride and preparation method and application thereof
A technology of pseudopolymorph and arbekacin, applied in the application field of arbekacin free base, can solve the problems of poor solid crystal form, inconvenient operation, limited purification effect, etc., and achieve thermodynamic stability and good crystal form , better purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
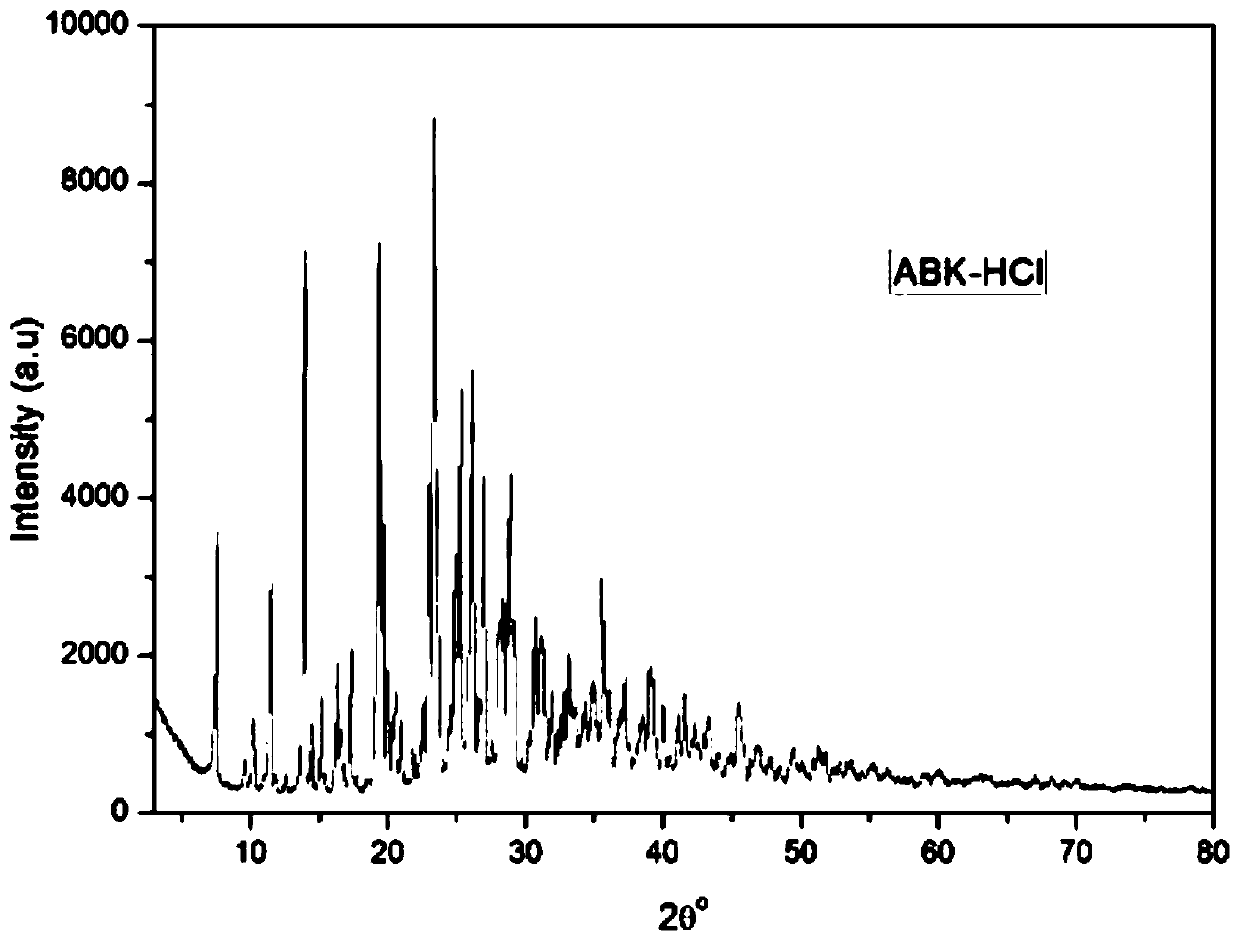
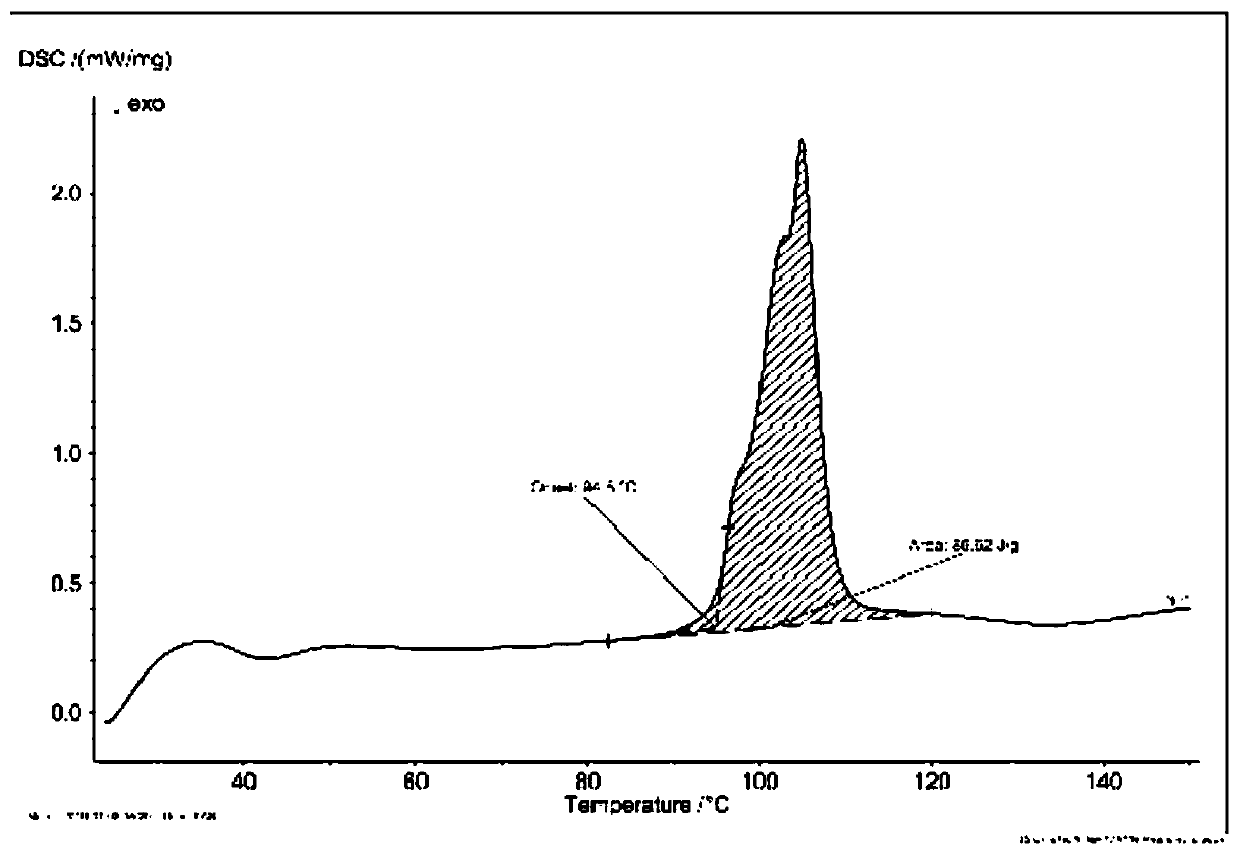
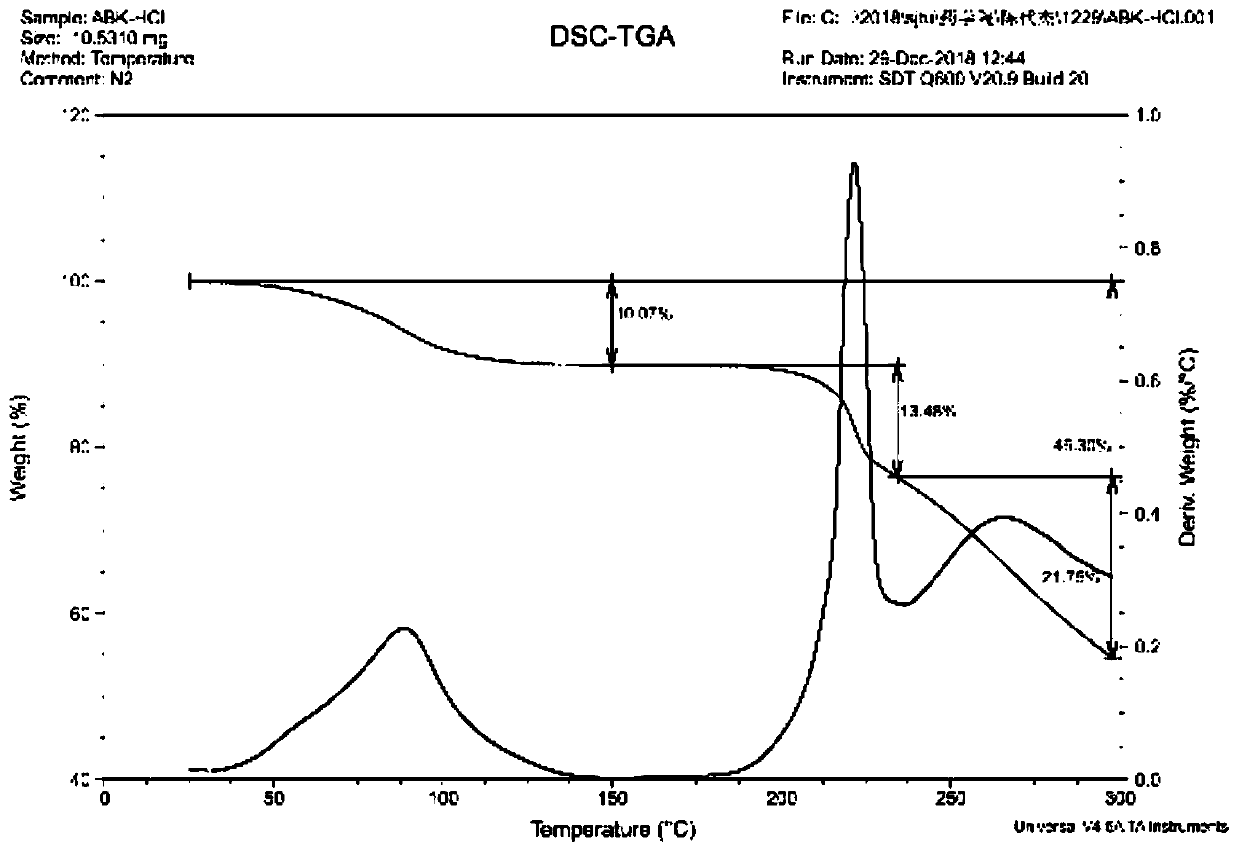
[0039] 5 g of arbekacin free base was dissolved in 15 mL of water and added to the reaction kettle. Add 6 mol / L hydrochloric acid aqueous solution dropwise to the reactor to adjust the final pH of the mother liquor to 3.0, and maintain the temperature at 30°C under stirring. Then add 100mL ethanol to the reaction kettle. After stirring for 3 hours, it was filtered to obtain 5.8 g of dry solid powder. The dried product was subjected to X-ray powder diffraction ( figure 1 ), DSC spectrum ( figure 2 ) and TGA spectrum ( image 3 ) was identified as pseudopolymorph II. The polymorph appears to have 2θ° of about 7.6, 9.6, 10.2, 11.5, 14.0, 14.5, 15.1, 16.3, 17.3, 19.3, 19.7, 20.0, 23.0, 23.4, 25.0, 25.3, 26.1, 27.0, 28.1, 28.9 , 33.2, 35.6, 37.2, 38.6, 39.0, 40.0, 41.0, 42.3, 43.3 and the X-ray powder diffraction pattern of the characteristic peaks represented by 45.5, such as figure 1 shown. The melting point of the pseudopolymorph II was measured to be 80-100°C.
Embodiment 2
[0041] 5 g of arbekacin free base was dissolved in 10 mL of water and added to the reaction kettle. Add 6 mol / L hydrochloric acid aqueous solution dropwise to the reaction kettle, adjust the final pH of the mother liquor to 4.0, and maintain the temperature at 30°C under stirring. Then add 70mL ethanol to the reaction kettle. After stirring for 3 hours, it was filtered to obtain 6.0 g of dry solid powder. The dried product was confirmed as pseudopolymorph II by X-ray powder diffraction, DSC spectrum and TGA spectrum.
Embodiment 3
[0043] 5 g of arbekacin free base was dissolved in 15 mL of water and added to the reaction kettle. Add 6 mol / L hydrochloric acid aqueous solution dropwise to the reaction kettle, adjust the final pH of the mother liquor to 4.5, and maintain the temperature at 30°C under stirring. 100 mL of methanol was then added to the reaction kettle. After stirring for 3 hours, it was filtered to obtain 5.7 g of dry solid powder. The dried product was confirmed as pseudopolymorph II by X-ray powder diffraction, DSC spectrum and TGA spectrum.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com