Ozone generating electrode, preparation method of electrode, and electrolytic device containing electrode
An ozone generation and electrode technology, which is applied in the field of electrochemistry, can solve the problems of difficulty in ozone entering water, low electrolysis efficiency and high use cost, and achieves the effects of good industrial application prospects, high ozone output and long service life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
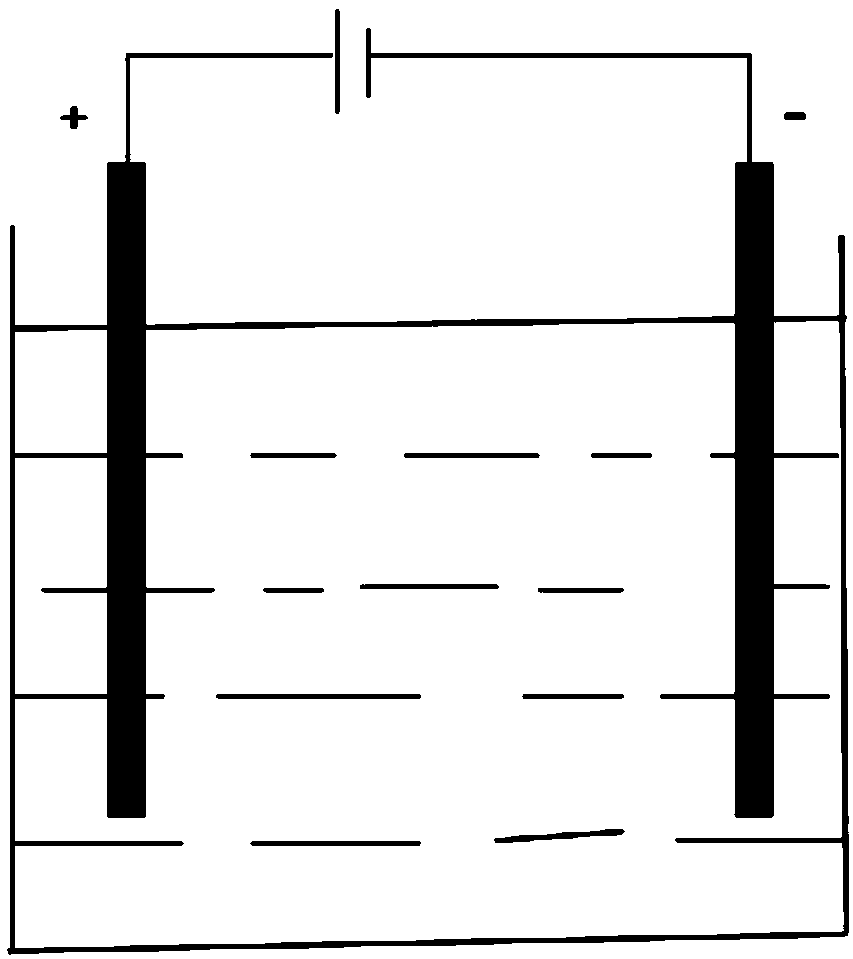
[0030] In view of the problems existing in the prior art, the present invention aims to provide a novel electrode, which comprises an electrode plate and a catalyst coated on the electrode plate. The present invention also provides a preparation method for the novel electrode, comprising the following steps:
[0031] A. Material preparation
[0032] Prepare electrode plates and catalyst:
[0033] (1) The electrode plate is made of non-metallic conductive material.
[0034] Wherein, the resistivity of the non-metallic conductive material selected in the present invention>1×10 -8 Ω·m (ohm meter). As a preferred embodiment of the present invention, the electrode plate is made of conductive ceramics or conductive silicon. More preferably, the conductive ceramics are silicon carbide ceramics, molybdenum disilicide ceramics, thorium oxide ceramics, and lanthanum chromate ceramics.
[0035] The non-metallic conductive material selected by the present invention has the following ...
Embodiment 1
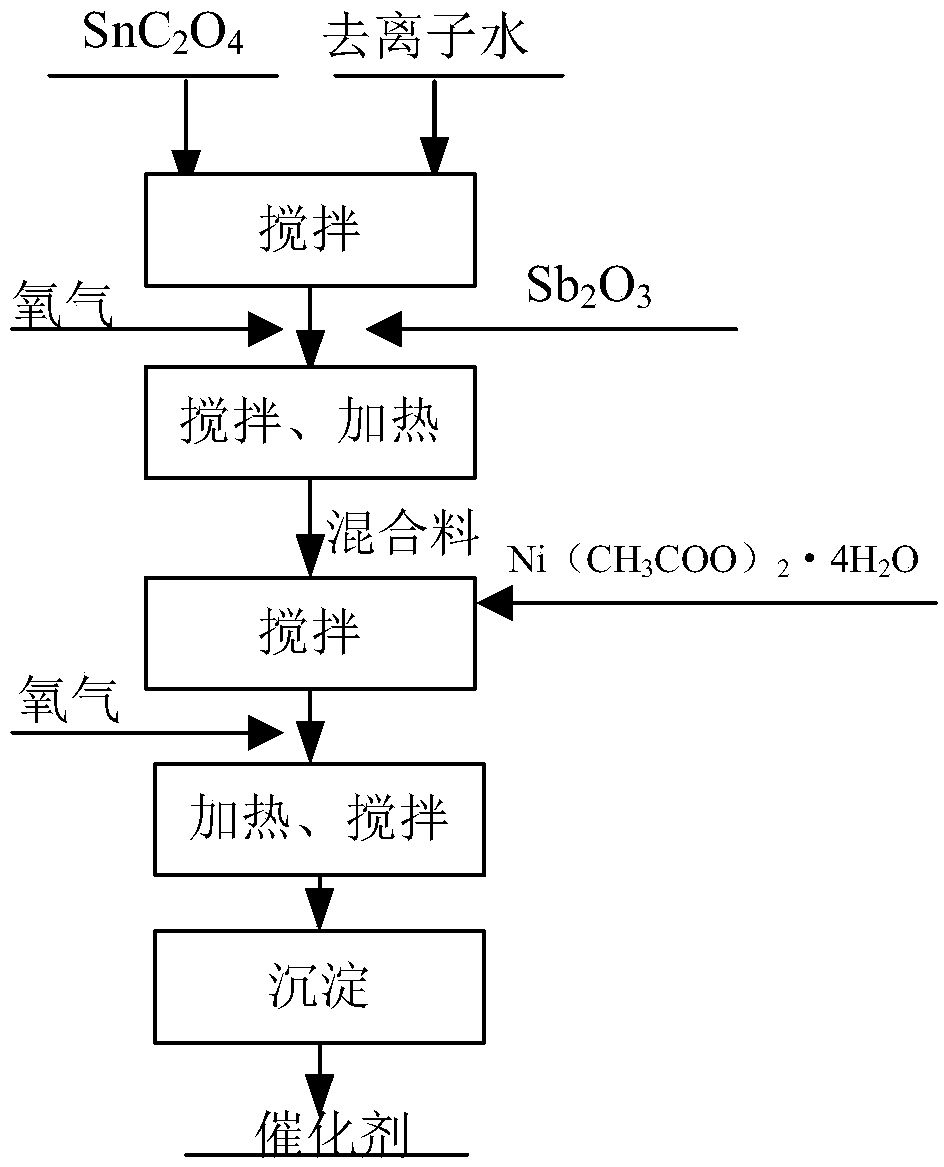
[0075] Take 850gSnC 2 o 4 Add to the reactor, add 200g deionized water to the reactor and stir for 3min, add 5gSb to the reactor 2 o 3 , stirred for 3 minutes, and oxygen was introduced into it at a flow rate of 10 L / min, and the reaction kettle was heated to a temperature of 50° C., and mixed evenly to obtain a mixture. Then add 1g Ni(CH 3 COO) 2 4H 2 O, then feed oxygen, the flow rate of oxygen is 10L / min, heat the reactor at 50°C for 2 hours, maintain the temperature in the reactor at 50°C during this period, and continue to feed oxygen for stirring until the material in the reactor In a suspended state. Finally, the materials in the reactor were allowed to cool naturally, and left to settle for 6 hours. After the precipitation was complete, the upper suspension was taken to obtain the catalyst.
[0076] Choose silicon carbide ceramics as the anode plate, coat the catalyst on the silicon carbide ceramics, pyrolyze the electrode plate at 500°C, repeat the coating-pyro...
Embodiment 2
[0081] Take 570gSnC 2 o 4 Add to the reactor, add 320g deionized water to the reactor and stir for 3min, add 4gSb to the reactor 2 o 3 , stirred for 3 minutes, and oxygen was passed into it at a flow rate of 12 L / min, and the reactor was heated to a temperature of 55° C., and mixed uniformly to obtain a mixture. Then add 3gNi(CH 3 COO) 2 4H 2 O, then feed oxygen, the flow rate of oxygen is 12L / min, heat the reactor at 55°C for 1.5h, maintain the temperature in the reactor at 55°C during this period, and continue to feed oxygen for stirring until the reactor The material is in a suspended state. Finally, the materials in the reactor were allowed to cool naturally, and left to settle for 8 hours. After the precipitation was complete, the upper suspension was taken to obtain the catalyst.
[0082] Silicon carbide ceramics were selected as the anode plate, the catalyst was coated on the silicon carbide ceramics, the electrode plate was pyrolyzed at 580°C, and the coating-py...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com