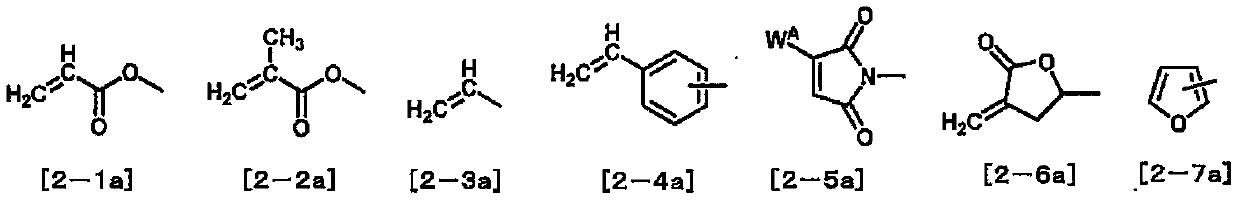
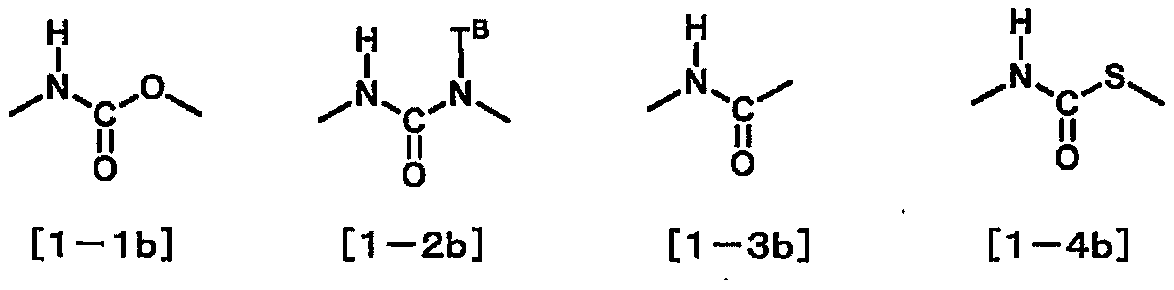
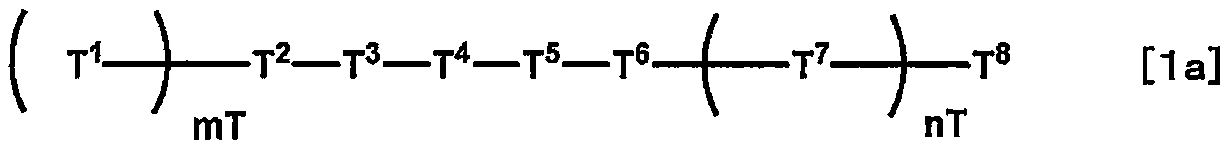Compound, liquid crystal composition and liquid crystal display element
A compound and liquid crystal technology, applied in the direction of liquid crystal materials, organic chemistry, chemical instruments and methods, etc., can solve problems such as increased power consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0278] The following examples are given to describe the present invention more specifically, but are not limited to these examples. Abbreviations used hereinafter are as follows.
[0279] "Compounds Used in Liquid Crystal Compositions"
[0280]
[0281]
[0282]
[0283]
[0284]
[0285] L1: MLC-6608 (manufactured by Merck & Co., Ltd.)
[0286]
[0287]
[0288] R3: BLEMMER TA-604AU (manufactured by NOF Corporation)
[0289]
[0290]
[0291] "Compounds used in liquid crystal aligning agents"
[0292]
[0293]
[0294]
[0295]
[0296]
[0297]
[0298]
[0299]
[0300]
[0301]
[0302]
[0303]
[0304] E2: Octadecyltriethoxysilane
[0305] E3: 3-methacryloxypropyltrimethoxysilane
[0306] E4: 3-Ureapropyltriethoxysilane
[0307] E5: Tetraethoxysilane
[0308]
[0309]
[0310]
[0311]
[0312]
[0313]
[0314]
[0315] NMP: N-methyl-2-pyrrolidone
[0316] γ-BL: γ-butyrolactone
[0317] BCS: Et...
Embodiment 2
[0413] Specific Compounds: Synthesis of T2
[0414]
[0415] At 25°C, compound (3) (5.01g, 17.9mmol), dibutylhydroxytoluene (0.010g, 0.046mmol), diazabicycloundecene (0.27g, 1.79mmol) and toluene (50g ), compound (2) (same as above) (4.69 g, 19.6 mmol) was added, and stirred at 110° C. for 48 hours. After completion of the reaction, dilute hydrochloric acid aqueous solution was added, and extraction and liquid separation were performed with chloroform. The chloroform layer was washed three times with dilute aqueous hydrochloric acid and twice with water, and dried by adding anhydrous magnesium sulfate. Thereafter, the solvent of the chloroform layer was distilled off under reduced pressure, and methanol (30 g) was added to the obtained residue. The precipitated solid was taken out by filtration and dried to obtain pale yellowish white crystals (T2) (yield: 4.69 g, yield: 50.5%).
[0416] 1H-NMR (CDCl 3 , σppm): 6.39-6.47 (m, 2H), 6.09-6.18 (m, 2H), 5.85-5.91 (m, 2H), 4....
Embodiment 3
[0418] Specific Compounds: Synthesis of T3
[0419]
[0420] At 25°C, in compound (4) (10.0g, 30.4mmol), dibutylhydroxytoluene (0.02g, 0.091mmol), diazabicycloundecene (0.46g, 3.04mmol) and toluene (100g ), compound (2) (same as above) (9.47 g, 39.6 mmol) was added, and stirred at 110° C. for 72 hours. After completion of the reaction, dilute hydrochloric acid aqueous solution was added, and extraction and liquid separation were performed with chloroform. The chloroform layer was washed three times with dilute aqueous hydrochloric acid and twice with water, and dried by adding anhydrous magnesium sulfate. Thereafter, the solvent of the chloroform layer was distilled off under reduced pressure, and isopropanol (150 g) was added to the obtained residue. Then, it heated to 40 degreeC and filtered. The obtained filtrate was distilled off under reduced pressure, methanol (150 g) was added to the residue, and the precipitated solid was collected by filtration. The obtained so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com