Application of chemotactic factor CCL8 in preparation of dermatomyositis condition and prognosis evaluation reagent
A prognostic assessment and chemokine technology, which can be used in disease diagnosis, biological testing, material testing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Sample selection and processing
[0024] Experimental group (DM): Take fresh peripheral blood from patients with dermatomyositis (DM) and centrifuge to separate the plasma, then quickly put it into a cryopreservation tube and store it in a -80°C refrigerator for later use;
[0025] Control group (HC): Fresh peripheral blood was collected from age- and gender-matched healthy people (HC) without immune diseases, and the plasma was separated by centrifugation, then quickly put into cryopreservation tubes, and stored in a -80°C refrigerator for later use.
[0026] 2. Screening and verification of plasma chemokines in patients with diabetes mellitus
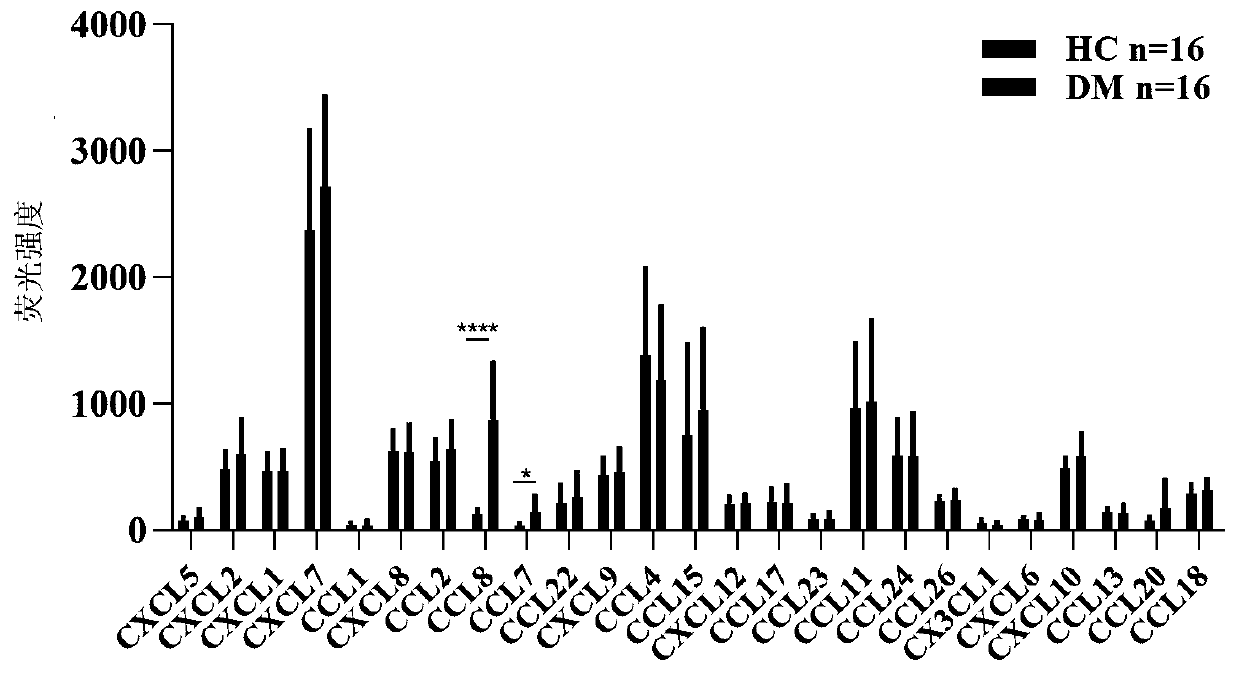
[0027] 2.1) Screening: In this experiment, we used the AAH-CYT-G5 protein chip purchased from RayBiotech to analyze the plasma samples of 16 healthy people and 16 patients with DM, and systematically compared and analyzed the plasma samples of HC and DM patients. Expression differences of various chemokines; by figure 1 Th...
Embodiment 2
[0051] A test kit for dermatomyositis condition and prognosis assessment, said test kit comprising:
[0052] a) container;
[0053] b) a specific antibody for the CCL8 protein contained in the container;
[0054] c) a label or an instruction for use, which indicates that the kit is used for evaluating the condition of dermatomyositis and using the kit to predict the prognosis of DM patients in the label or the instruction for use.
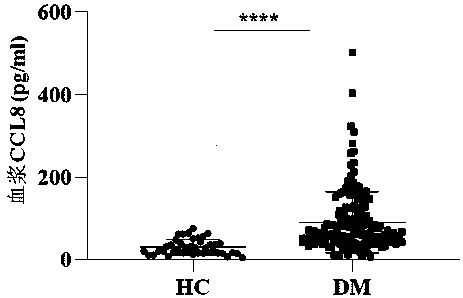
[0055] Further, the label or instructions for use indicate: when the plasma CCL8 value of a DM patient is ≥67.3pg / ml, the patient is diagnosed as having a severe condition and poor prognosis; when the plasma CCL8 value of a patient with dermatomyositis is <67.3pg / ml, The patient was diagnosed with a mild condition and a good prognosis.
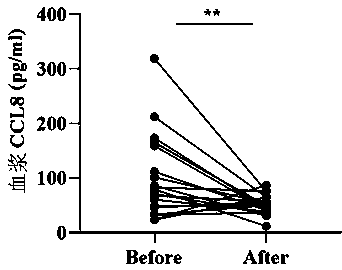
[0056] Utilize above-mentioned detection kit of the present invention, quantitatively detect 129 routine DM patient's plasma samples (with DM characteristic erythra (Xiangyang rash or Gottron sign) appearing for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com