Boron-doped silver nano spongy catalyst for electrochemical ammonia synthesis and preparation method thereof
A catalyst and sponge-like technology, which is applied in the field of boron-doped silver nano-sponge electrochemical ammonia synthesis catalyst and its preparation, can solve the problems of low NH3 Faraday efficiency and cannot be applied in industrial fields, and achieve high yield, mild preparation method, The effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A preparation method of a boron-doped silver nano-sponge electrochemical catalyst for ammonia synthesis, said method comprising the steps of:
[0036] (1) prepare the DMF solution that concentration is the silver nitrate of 0.1M and sodium borohydride respectively;
[0037] (2) get 1.0mL concentration and be the DMF solution of the silver nitrate of 0.1M, join then 5.0mL concentration and be the DMF solution of the sodium borohydride of 0.1M, stir reaction 1.5 hours under ice-water bath condition, wash, centrifuge, dry, The boron-doped silver nano-sponge catalyst is obtained.
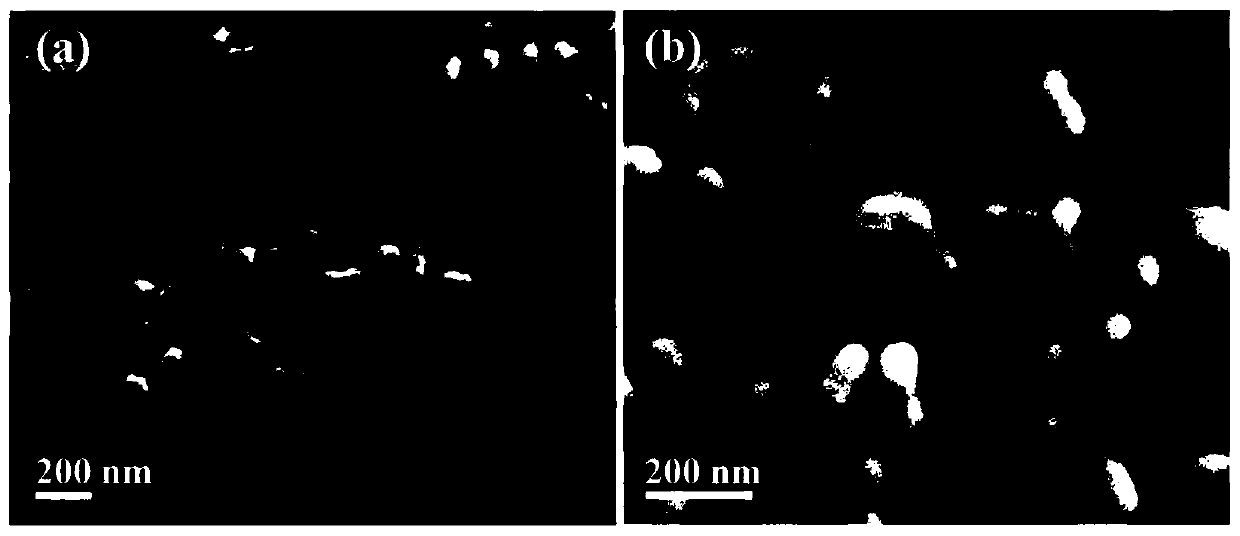
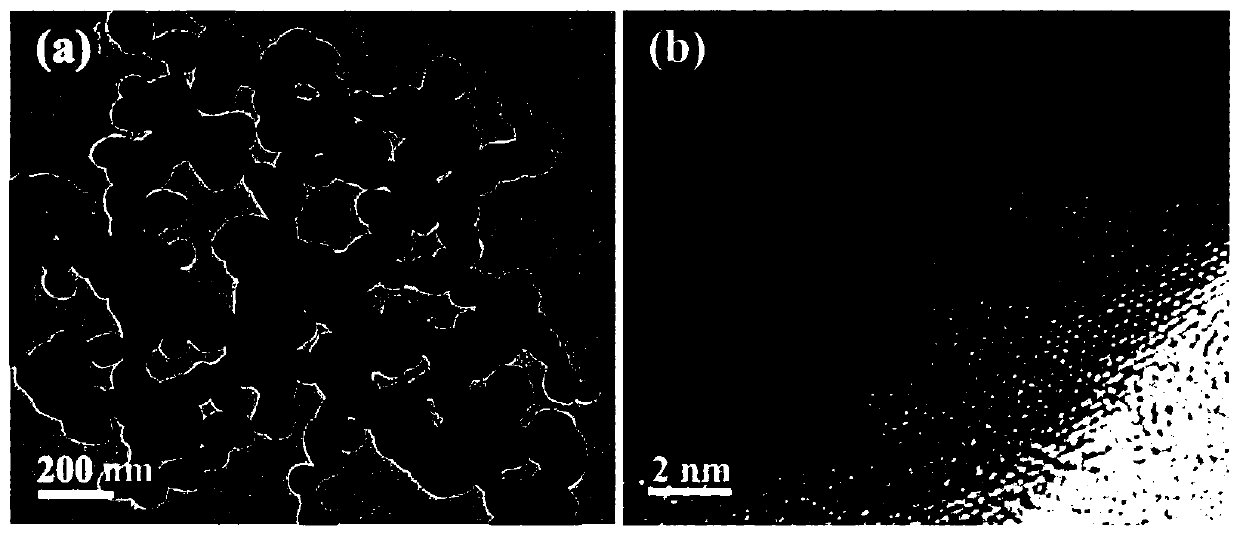
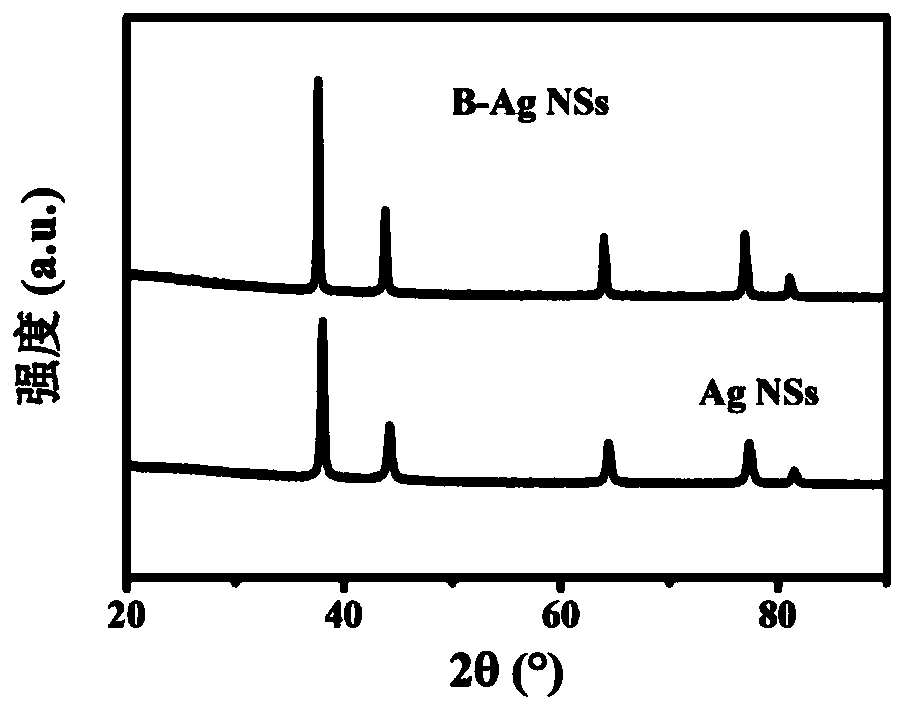
[0038] The SEM figure of the obtained boron-doped silver nano-sponge electrochemical ammonia synthesis catalyst can be found in figure 1 . The TEM picture of obtained boron-doped silver nano-sponge electrochemical ammonia synthesis catalyst can be found in figure 2 . The XRD pattern of obtained boron-doped silver nano-sponge electrochemical ammonia synthesis catalyst can be found in image ...
Embodiment 2
[0041] A preparation method of a silver nano-sponge electrochemical ammonia synthesis catalyst, said method comprising the steps of:
[0042] (1) prepare the aqueous solution of silver nitrate and sodium borohydride that concentration is 0.1M respectively;
[0043] (2) Get 1.0mL concentration and be the aqueous solution of silver nitrate of 0.1M, then join into 5.0mL concentration and be the aqueous solution of 0.1M sodium borohydride, stir reaction 2 hours under ice-water bath condition, wash, centrifuge, dry, obtain obtained Described silver nano-sponge catalyst.
[0044] Obtain the SEM figure of silver nano-sponge catalyst see Figure 7 ; Silver nano-sponge catalyst catalyzes the performance diagram of nitrogen reduction to prepare ammonia, see Figure 8 .
[0045] It can be seen from the SEM image that the silver nano-sponge electrochemical ammonia synthesis catalyst has been formed. The performance diagram of ammonia preparation by nitrogen reduction shows that the am...
Embodiment 3
[0047] A method for preparing boron-doped silver nanoparticles, the method comprising the steps of:
[0048] (1) prepare the DMF solution that concentration is the silver nitrate of 0.5M and sodium borohydride respectively;
[0049] (2) get 1.0mL concentration and be the DMF solution of the silver nitrate of 0.5M, then add to 5.0mL concentration and be the DMF solution of the sodium borohydride of 0.5M, stir reaction 2.5 hours under ice-water bath condition, wash, centrifuge, dry, The boron-doped silver nanoparticle catalyst is obtained.
[0050] Obtain the SEM image of boron-doped silver nanoparticles see Figure 9 .
[0051] It can be seen from the SEM images that boron-doped silver nanoparticles have been formed. This is mainly due to the change in the morphology of the boron-doped silver due to changing the concentration of the precursor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com