A kind of preparation method of afatinib maleate
A technology of afatinib maleate and afatinib acid is applied in the field of pharmaceutical preparation, which can solve the problems of lowering yield and quality, the existence of impurities in the product afatinib maleate, and achieving no solvent residue, The effect of stable properties and low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, the preparation of Afatinib maleate
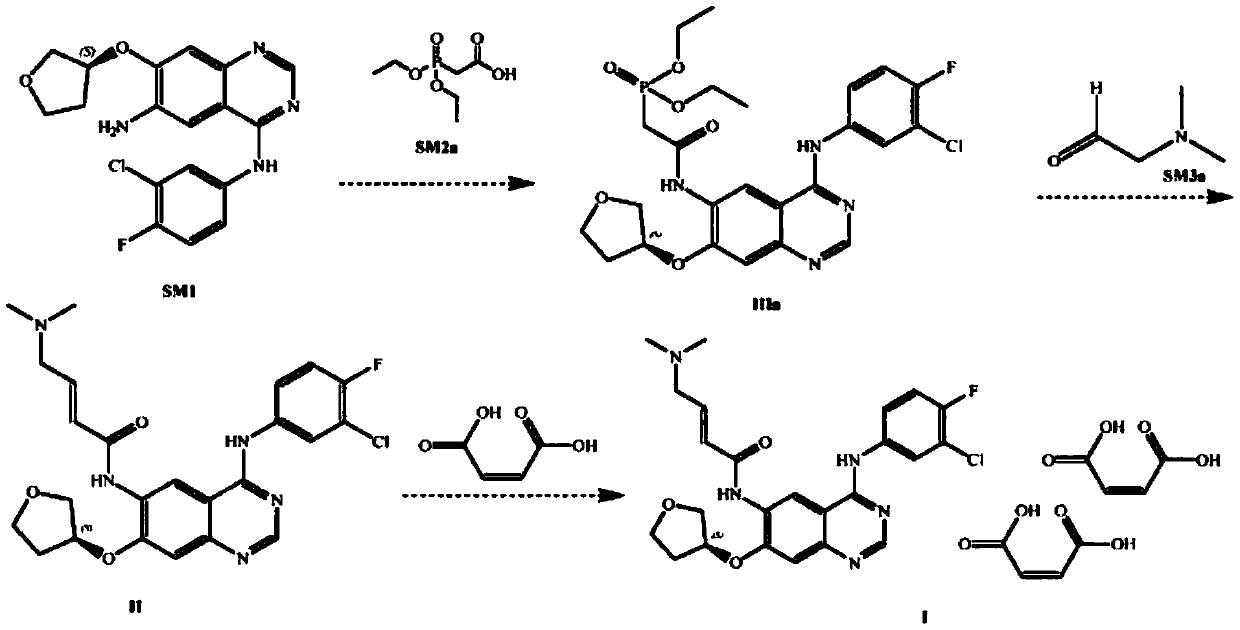
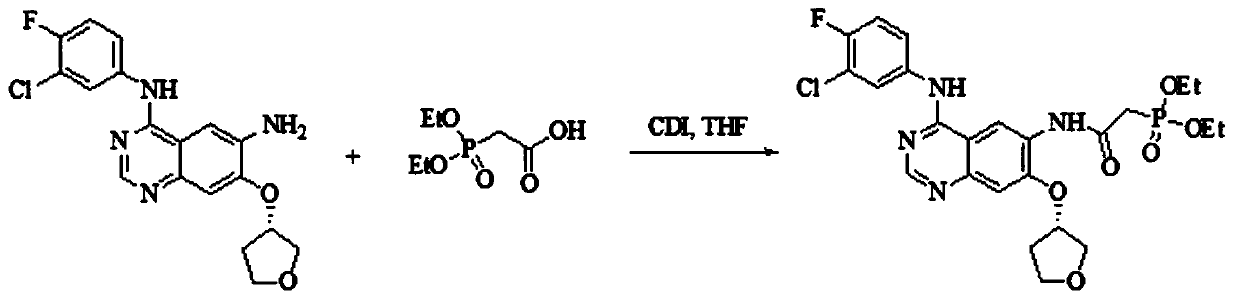
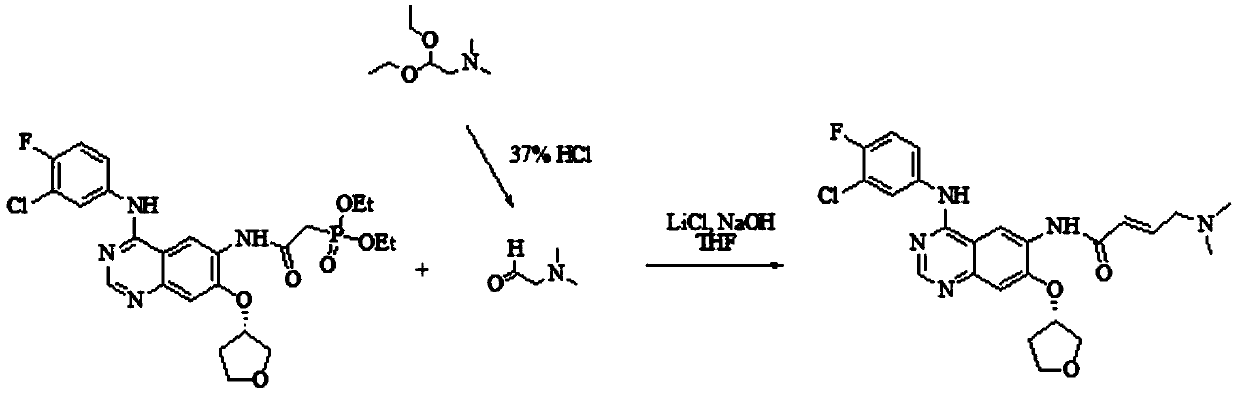
[0027] S1) Preparation of Intermediate I
[0028] The chemical reaction equation is:
[0029]
[0030] Take 3.58 L of tetrahydrofuran and N,N-carbonyldiimidazole (1433 g, 8.84 mol) and mix and stir at room temperature, slowly add diethylphosphonoacetic acid (1734 g, 8.84 mol) in 2.43 L of tetrahydrofuran solution, complete the addition in 30 minutes, and control the temperature for 40 ℃ and stirred for 30min until the solution was clear to obtain reaction solution A; 7.65L of tetrahydrofuran and N 4 -(3-Chloro-4-fluorophenyl)-7-[[(3S)-tetrahydro-3-furyl]oxy]-4,6-quinazolinediamine (2550g, 6.8mol) was added to In reaction kettle A, stir at room temperature, add the above-mentioned reaction solution A, and stir at 40°C for 1 hour. After the completion of the reaction is monitored by thin-layer chromatography, cool down to 8°C, filter, and dry to obtain intermediate I.
[0031] Among them, thin-layer chromatography...
Embodiment 2~3
[0042] Embodiment 2~3, the preparation of Afatinib maleate
[0043] The difference from Example 1 is that the amount of raw materials used in Examples 2-3 is different, see Table 1 for details.
[0044] Table 1 embodiment 2~3 formula (AF-I) afatinib maleate intermediate raw material feeding amount
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com