Oocyte in-vitro maturation culture solution and application thereof
An in vitro mature culture and oocyte technology, applied in the direction of cell culture active agent, germ cells, culture process, etc., can solve the problems of unclear composition of follicle fluid, unsatisfactory culture efficiency, etc., and achieve better results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
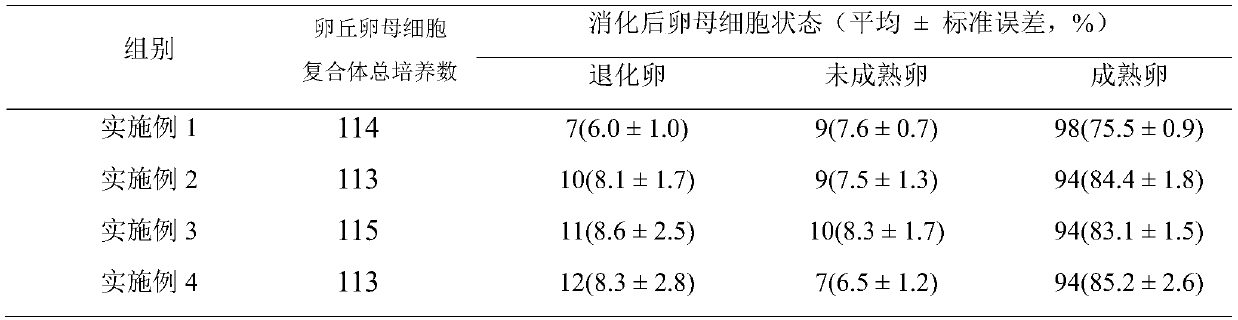
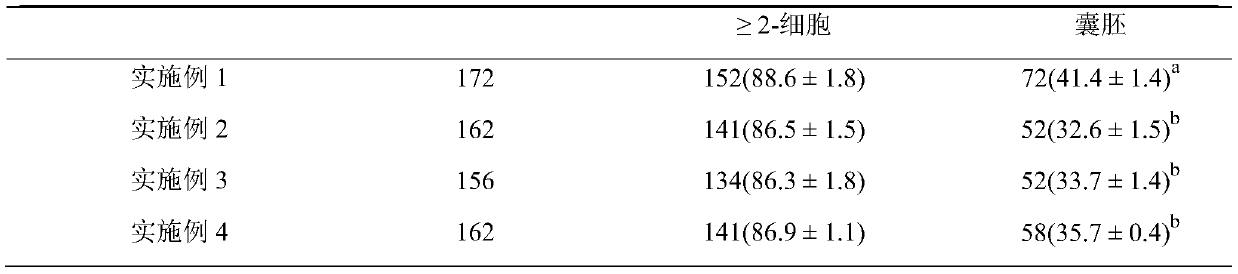
[0014] Embodiment 1. Oocyte maturation medium in vitro
[0015] The specific components of the oocyte maturation medium in vitro described in the present embodiment are as follows:
[0016] Based on TCM-199, the volume fraction of KSR is 50mL / L, the mass concentration of penicillin is 0.06g / L, the mass concentration of streptomycin is 0.1g / L, and the mass concentration of polyvinyl alcohol is 3g / L. The mass concentration of insulin is 10ng / mL, the mass concentration of epidermal growth factor is 10ng / mL, the concentration of sodium pyruvate is 0.91mmol / L, the concentration of NaHCO3 is 1.99mmol / L, and the concentration of Hepes is 9.8mmol / L. The concentration of cystine was 0.6mmol / L, the concentration of follicle growth hormone was 10IU / mL, and the concentration of luteinizing hormone was 10IU / mL.
Embodiment 2
[0017] Embodiment 2. Oocyte maturation medium in vitro
[0018] The specific components of the oocyte maturation medium in vitro described in the present embodiment are as follows:
[0019] Based on TCM-199, the volume fraction of KSR is 100mL / L, the mass concentration of penicillin is 0.048g / L, the mass concentration of streptomycin is 0.12g / L, and the mass concentration of polyvinyl alcohol is 2.5g / L , the mass concentration of insulin is 12ng / mL, the mass concentration of epidermal growth factor is 8ng / mL, the concentration of sodium pyruvate is 0.95mmol / L, NaHCO 3 The concentration of Hepes is 1.95mmol / L, the concentration of Hepes is 10mmol / L, the concentration of cysteine is 0.5mmol / L, the concentration of follicle growth hormone is 12IU / mL, and the concentration of luteinizing hormone is 8IU / mL.
Embodiment 3
[0020] Embodiment 3. Oocyte maturation medium in vitro
[0021] The specific components of the oocyte maturation medium in vitro described in the present embodiment are as follows:
[0022] Based on TCM-199, the volume fraction of KSR is 75mL / L, the mass concentration of penicillin is 0.066g / L, the mass concentration of streptomycin is 0.08g / L, and the mass concentration of polyvinyl alcohol is 3.5g / L , the mass concentration of insulin is 8ng / mL, the mass concentration of epidermal growth factor is 12ng / mL, the concentration of sodium pyruvate is 0.9mmol / L, NaHCO 3 The concentration of Hepes is 2mmol / L, the concentration of Hepes is 9.5mmol / L, the concentration of cysteine is 0.7mmol / L, the concentration of follicle growth hormone is 8IU / mL, and the concentration of luteinizing hormone is 12IU / mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com