Novel quinazolinone compound as well as preparation method and application thereof
A quinazolinone and compound technology, applied in the field of novel quinazolinone compounds and their preparation, can solve the problems of high onset concentration, unclear mode of action, and small quantity, and achieve DNA damage induction and normal cell toxicity Small, strong selective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
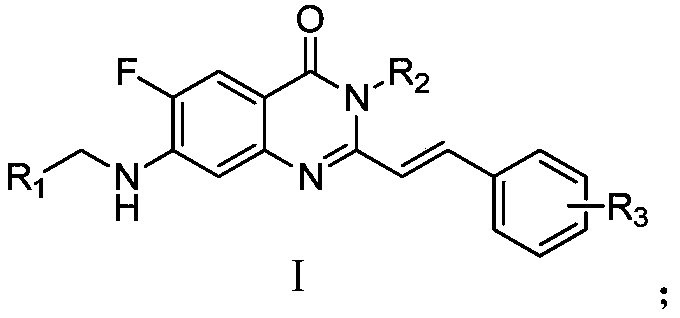
[0042] Example 1 Preparation of compound 1f
[0043] The preparation process of compound 1f is as follows:
[0044] S1, synthesis of intermediate d1
[0045]
[0046] 4.2 g (24.3 mmol) of 2-amino-4,5-difluorobenzoic acid was added to a 100 mL reaction flask containing 12 mL of acetic anhydride, and reacted at 120° C. for 1.5 hours. After cooling, a large amount of white solid precipitated. The reaction solution was rotary evaporated under reduced pressure to remove most of the acetic anhydride, and a large amount of white solid appeared, which was filtered with suction and washed with ethanol to obtain a white solid d1, which was directly used for the next reaction.
[0047] 1 H NMR (400MHz, DMSO) δ 8.13 (dd, J = 9.9, 8.5 Hz, 1H), 7.74 (dd, J = 11.1, 7.2 Hz, 1H), 2.40 (s, 3H).
[0048] S2, the synthesis of intermediate d2
[0049]
[0050] Take 1g of d1 (5.07mmol) and 9mL of ammonia in a 100mL reaction flask, condense and reflux at 70°C (a balloon is placed on the condenser to prevent ...
Embodiment 2
[0079] Example 2 EMSA verification helicase activity experiment
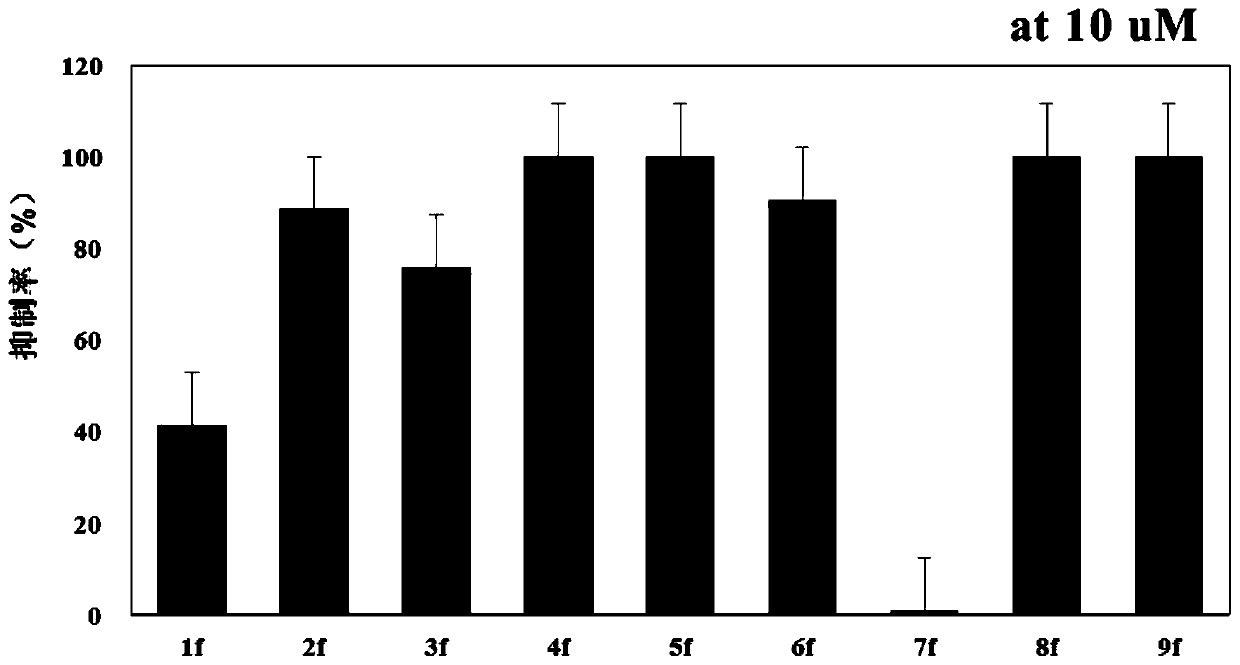
[0080] The compound prepared in Example 1 was used as the test object to test its ability to inhibit the unwinding of BLM.
[0081] 1. Anneal the double-stranded Biotin forked-DNA with a final concentration of 10nM at 95°C for 5min and slowly lower the temperature to room temperature to form a stable double helix structure;
[0082] 2. Mix the purified BLM protein and the compounds of different concentrations in the helicase working solution, and incubate them at 37°C for 1 h. The final concentration of the protein is 30 nM, and then mix the protein compound solution with the DNA solution, and mix. After homogenization, continue to incubate at 37℃ for 1h;
[0083] 3. After the incubation, add DNA Loading buffer to the sample to end the enzymatic reaction. After mixing well, load the sample to 8% Native-PAGE, buffer 0.5×TB, 80V ice bath for about 3h, to bromine The phenol blue band is close to the edge of the electropho...
Embodiment 3
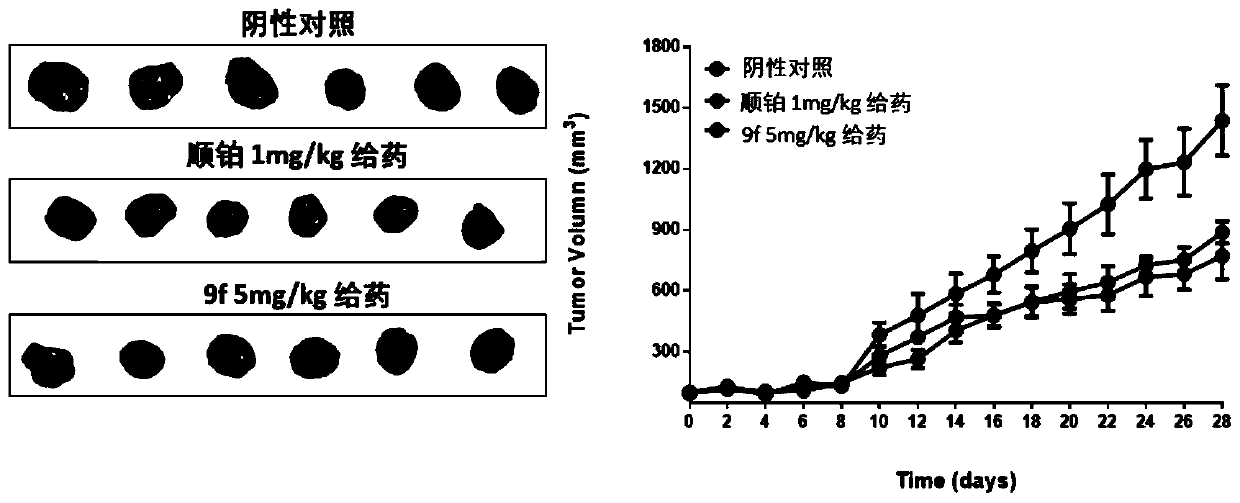
[0087] Example 3 MTT experiment
[0088] 1. Inoculate HCT116 cells in logarithmic growth phase on a 96-well cell culture plate with 5000 cells / well, and place them in an incubator containing 5% CO2 for 24 hours;
[0089] 2. After the cells are completely attached to the wall, discard the old medium, add medium containing different concentrations of compounds, and culture for different times according to the requirements of different experiments;
[0090] 3. When testing, add 20μL of MTT solution with a concentration of 2.5mg / mL to each well of cells and continue to incubate at 37°C for 4h;
[0091] 4. After the MTT incubation, discard the old culture medium and add 100μL of DMSO to each well. At this time, the solution in the well is purple. After shaking evenly, use a multifunctional microplate reader to detect the absorption value of each well at 570nm wavelength, and obtain the half inhibitory concentration IC of the compound on cell proliferation according to the relationship betw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com