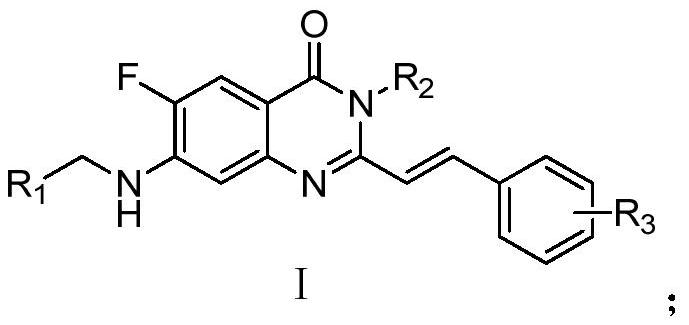
A novel quinazolinone compound and its preparation method and application
A quinazolinone and compound technology, applied in the field of novel quinazolinone compounds and their preparation, can solve the problems of high onset concentration, unclear mode of action, and small quantity, and achieve DNA damage induction and normal cell toxicity Small, strong selective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
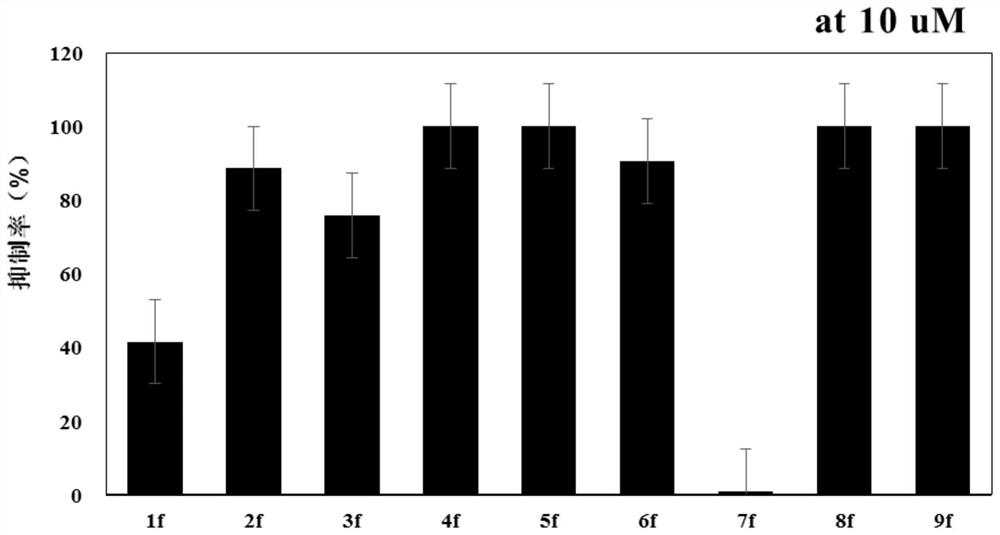
Examples
Embodiment 1
[0042] The preparation of embodiment 1 compound 1f
[0043] The preparation process of compound 1f is specifically as follows:
[0044] S1, synthesis of intermediate d1
[0045]
[0046] Add 4.2g (24.3mmol) of 2-amino-4,5-difluorobenzoic acid into a 100mL reaction flask filled with 12mL of acetic anhydride, and react at 120°C for 1.5 hours. After cooling, a large amount of white solid precipitated out. The reaction liquid was rotary-evaporated under reduced pressure. After removing most of the acetic anhydride, a large amount of white solid appeared, which was filtered by suction and washed with ethanol to obtain the white solid d1, which was directly put into the next reaction.
[0047] 1 H NMR (400MHz, DMSO) δ8.13(dd, J=9.9, 8.5Hz, 1H), 7.74(dd, J=11.1, 7.2 Hz, 1H), 2.40(s, 3H).
[0048] S2, synthesis of intermediate d2
[0049]
[0050] Take 1g of d1 (5.07mmol) and 9mL ammonia water in a 100mL reaction flask, condense and reflux at 70°C (put a balloon on the cond...
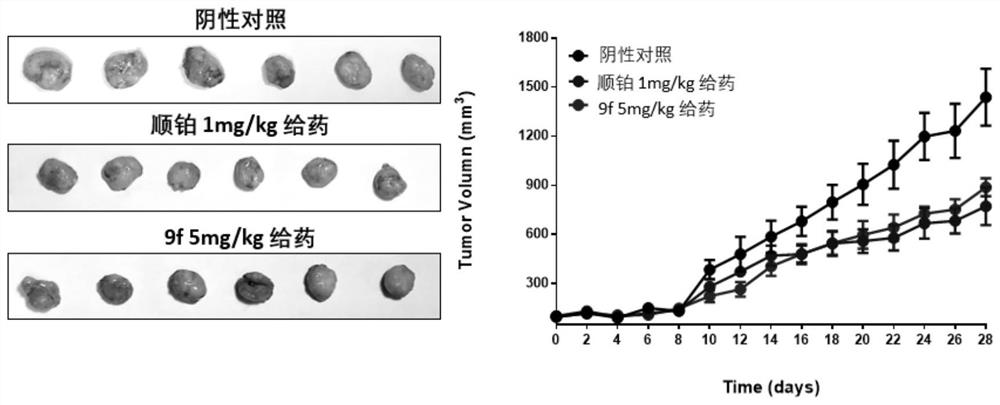
Embodiment 2
[0079] Example 2 EMSA verification experiment of helicase activity
[0080] The compound prepared in Example 1 was used as the test object to test its ability to inhibit the unwinding of BLM.
[0081] 1. Anneal double-stranded Biotin forked-DNA with a final concentration of 10 nM at 95°C for 5 minutes and slowly cool down to room temperature to form a stable double helix structure;
[0082] 2. Mix the purified BLM protein with different concentrations of compounds in the helicase working solution, and incubate at 37°C for 1 hour. The final concentration of the protein is 30nM, then mix the protein compound mixed solution with the DNA solution, and mix After homogenization, continue to incubate at 37°C for 1 hour;
[0083] 3. After the incubation, add DNA Loading buffer to the sample to end the enzyme reaction. After mixing well, load the sample to 8% Native-PAGE with a buffer of 0.5×TB, 80V ice bath for about 3 hours, until bromine The phenol blue band is close to the edge o...
Embodiment 3
[0087] Embodiment 3 MTT experiment
[0088] 1. Inoculate the HCT116 cells in the logarithmic growth phase in a 96-well cell culture plate, the number of cells is 5000 / well, and place them in a 5% CO2 incubator for 24 hours;
[0089] 2. After the cells are completely adhered to the wall, discard the old medium, add medium containing different concentrations of compounds, and culture for different times according to the requirements of different experiments;
[0090] 3. When testing, add 20 μL of MTT solution with a concentration of 2.5 mg / mL to each well of cells, and continue to incubate at 37 ° C for 4 h;
[0091] 4. After MTT incubation, discard the old culture medium, add 100 μL of DMSO to each well, and the solution in the well is purple at this time. After oscillating evenly, use a multi-functional microplate reader to detect the absorption value of each well at a wavelength of 570nm, and calculate the half inhibitory concentration IC of the compound on cell proliferation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com