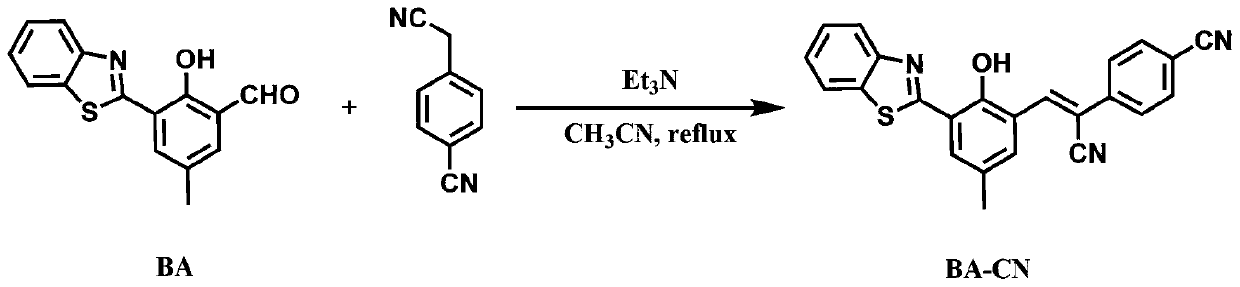
Benzothiazole-benzyl cyanide compound and preparation method and application thereof
A technology of benzothiazole and phenethyl cyanide, applied in chemical instruments and methods, organic chemistry, fluorescence/phosphorescence, etc., can solve the problems of short fluorescence emission wavelength, difficult synthesis, small Stokes shift, etc., and achieve convenient purification. , broad application prospects, the effect of large Stokes displacement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
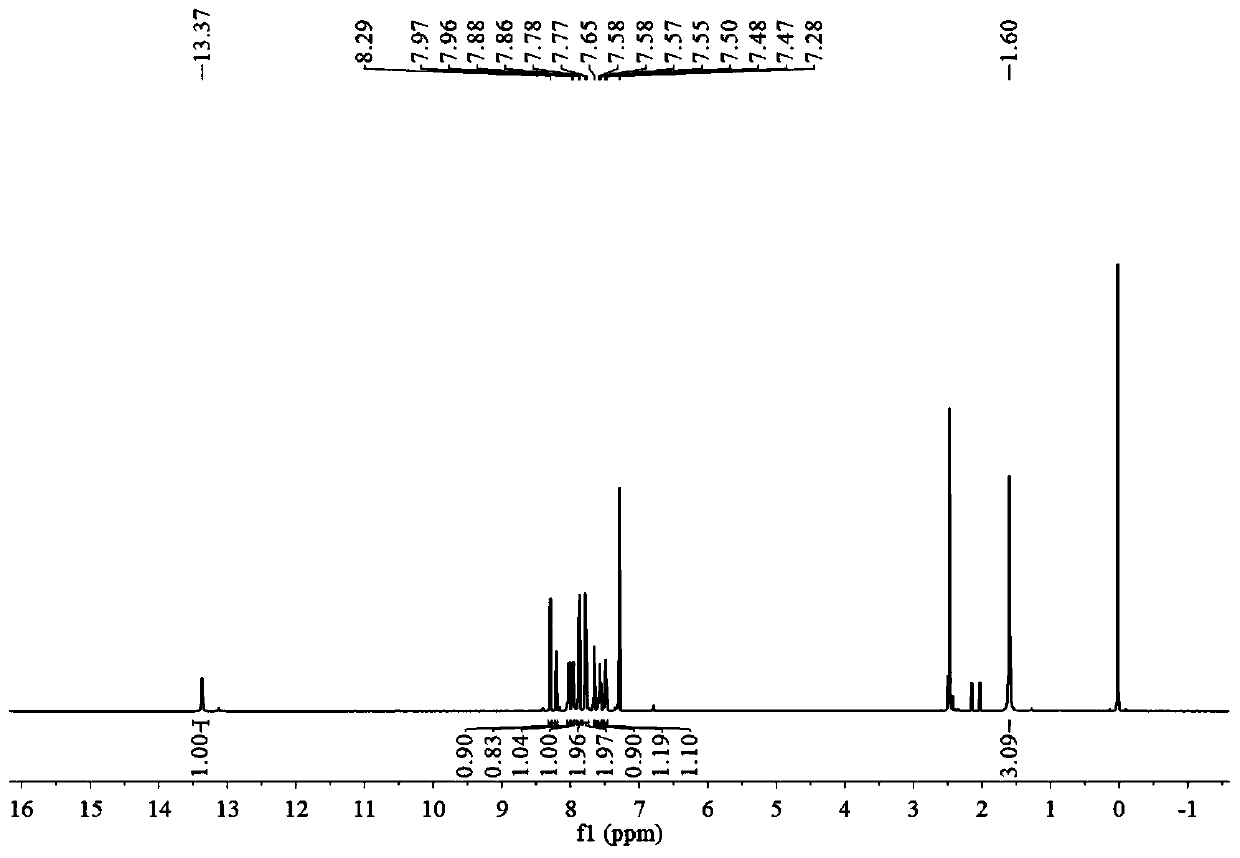
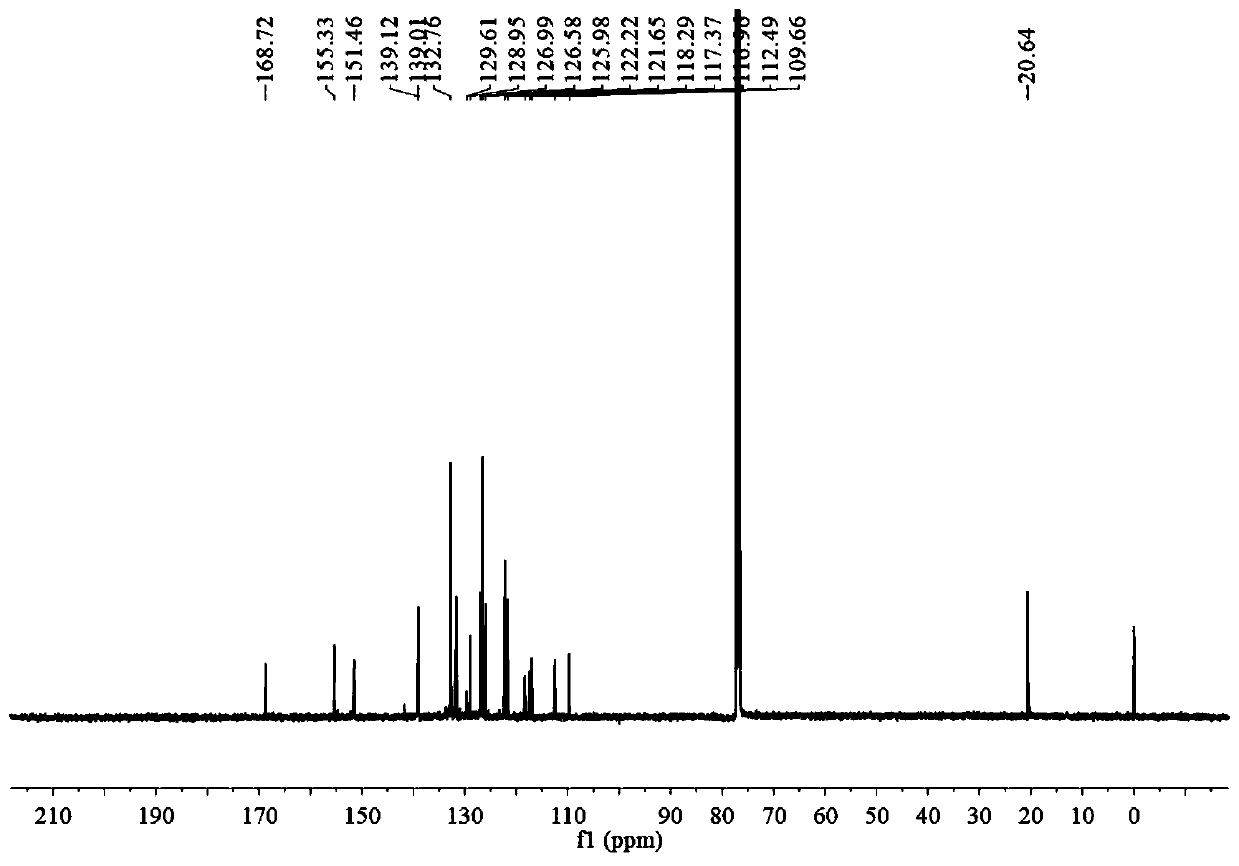
[0043] Take a clean round bottom flask, add 1 mmol of BA, 71.1 mg of 4-cyanophenylacetonitrile, 100 μl of triethylamine and 25 ml of acetonitrile in sequence, and stir at 82°C for 12 hours. After the reaction was finished, a brown precipitate was precipitated, and TLC plate was spotted, and it was found that most of the raw material BA was unreacted. The precipitate was filtered and washed 2-3 times with acetonitrile to obtain pure BA-CN (yield 41.6%).
Embodiment 2
[0045] Take a clean round bottom flask, add 1 mmol BA, 142.2 mg 4-cyanophenylacetonitrile, 100 μl triethylamine and 25 ml acetonitrile in sequence, and stir at 82°C for 12 hours. After the reaction was finished, a brown precipitate precipitated out, and TLC spotting showed that the raw material BA was almost completely reacted. The precipitate was filtered and washed 2-3 times with acetonitrile to obtain pure BA-CN (yield 86.2%).
Embodiment 3
[0047] Take a clean round bottom flask, add 1 mmol of BA, 284.3 mg of 4-cyanophenylacetonitrile, 100 μl of triethylamine and 25 ml of acetonitrile in sequence, and stir at 82°C for 12 hours. After the reaction was finished, a brown precipitate precipitated out, and TLC spotting showed that the reaction of raw material BA was complete, and a large amount of 4-cyanophenylacetonitrile remained. The precipitate was filtered, washed with acetonitrile for 2-3 times, and then separated by column chromatography to obtain pure BA-CN (71.6% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com