A kind of solid dispersion method of celecoxib and the preparation method of celecoxib capsule
A technology of celecoxib and capsules, which is applied in the field of pharmaceutical preparations, can solve the problems of celecoxib, such as serious wall adhesion, difficulty in crushing, and agglomeration, and achieve the effects of fast and convenient detection, short crushing time, and consistent dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
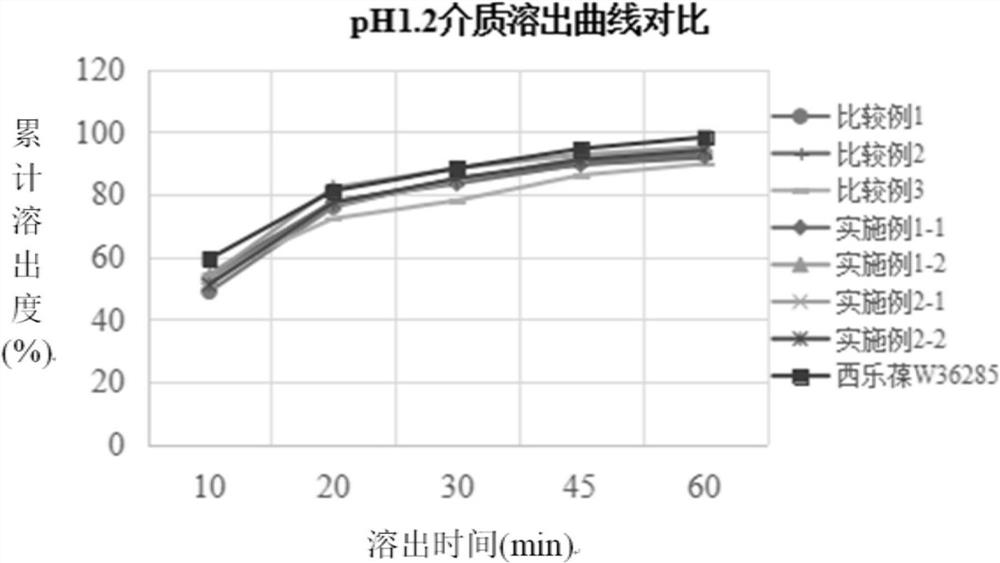
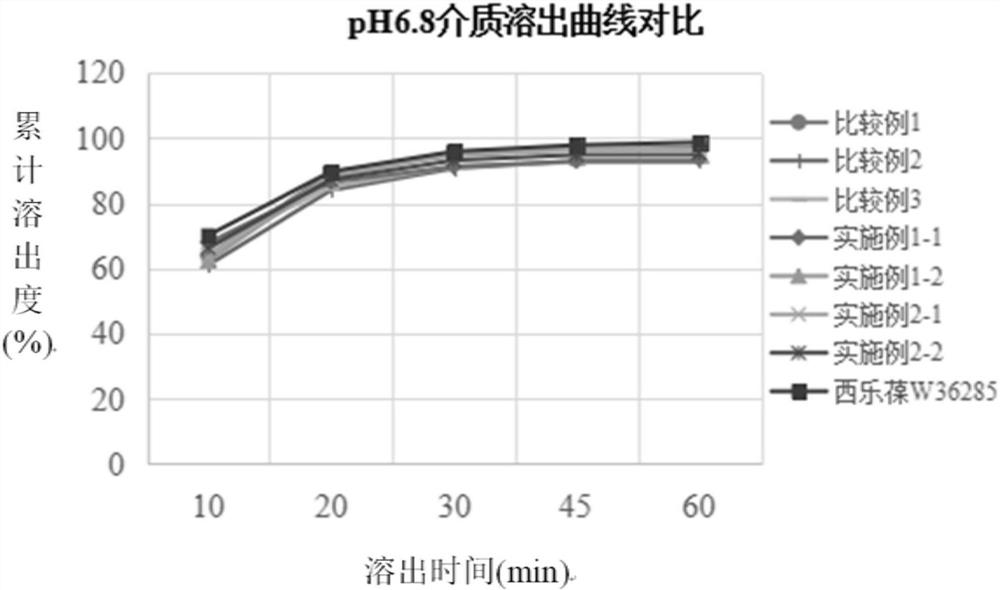
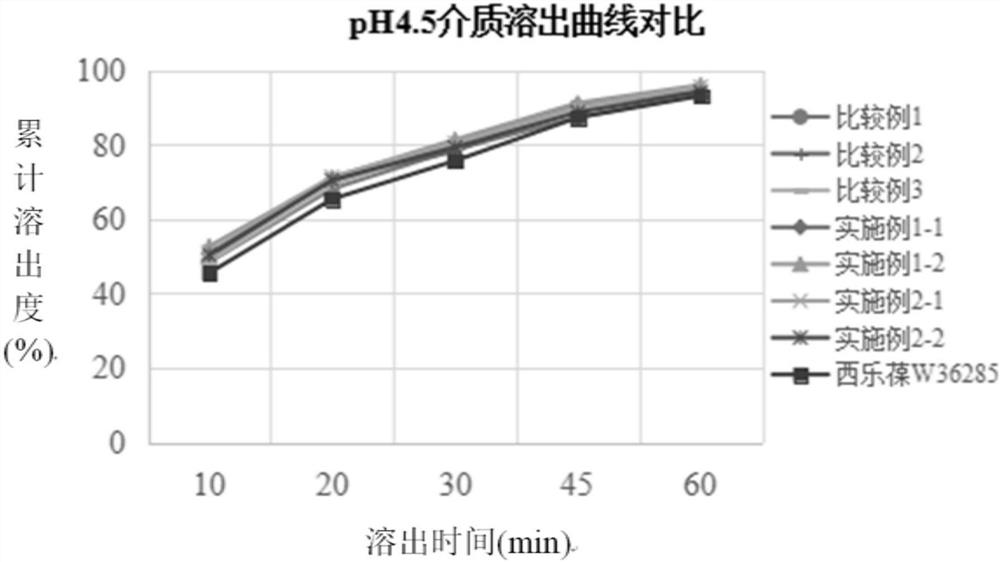
[0034] The preparation method of the present disclosure not only solves the problems that celecoxib is easy to agglomerate into agglomerates, has serious wall adhesion and is difficult to crush, but also solves the problem of slow dissolution.
[0035] In one or more examples of this embodiment, the particle size D of the pulverized solid material 90 ≤20μm.
[0036] In one or more examples of this embodiment, the pharmaceutical excipients include polyvinylpyrrolidone and croscarmellose sodium.
[0037] In this series of examples, the mass ratio of celecoxib, lactose monohydrate, sodium lauryl sulfate, polyvinylpyrrolidone, and croscarmellose sodium is 30~40:50~60:1~5: 1~5: 0.5~5.
[0038]During the powder mixing process of raw materials and excipients in the present disclosure, a part of the selected drug excipients can be added in part or in whole. For example, when lactose monohydrate and sodium lauryl sulfate are selected to be mixed with celecoxib, the It can be: lactos...
Embodiment 1
[0049] Celecoxib, lactose monohydrate and sodium lauryl sulfate ultrafine airflow pulverization solid dispersion process: weigh celecoxib, lactose monohydrate and sodium lauryl sulfate according to the prescription ratio and mix them for later use; Excipients are mixed with powder for ultra-fine airflow pulverization and solid dispersion to control particle size D 90 ≤20μm, then wet granulate with polyvinylpyrrolidone and croscarmellose sodium in the prescription ratio, dry, and granulate; mix the obtained granules with magnesium stearate in the prescription ratio, and fill it to obtain the plug Lecoxib capsules.
Embodiment 2
[0051] Mechanical pulverization of celecoxib, lactose monohydrate and sodium lauryl sulfate solid dispersion process: weigh celecoxib, lactose monohydrate and sodium lauryl sulfate according to the prescription ratio and mix them for later use; mix the raw and auxiliary materials The powder is mechanically pulverized and solid dispersed to control the particle size D 90 ≤20μm, then wet granulate with polyvinylpyrrolidone and croscarmellose sodium in the prescription ratio, dry, and granulate; mix the obtained granules with magnesium stearate in the prescription ratio, and fill it to obtain the plug Lecoxib capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com