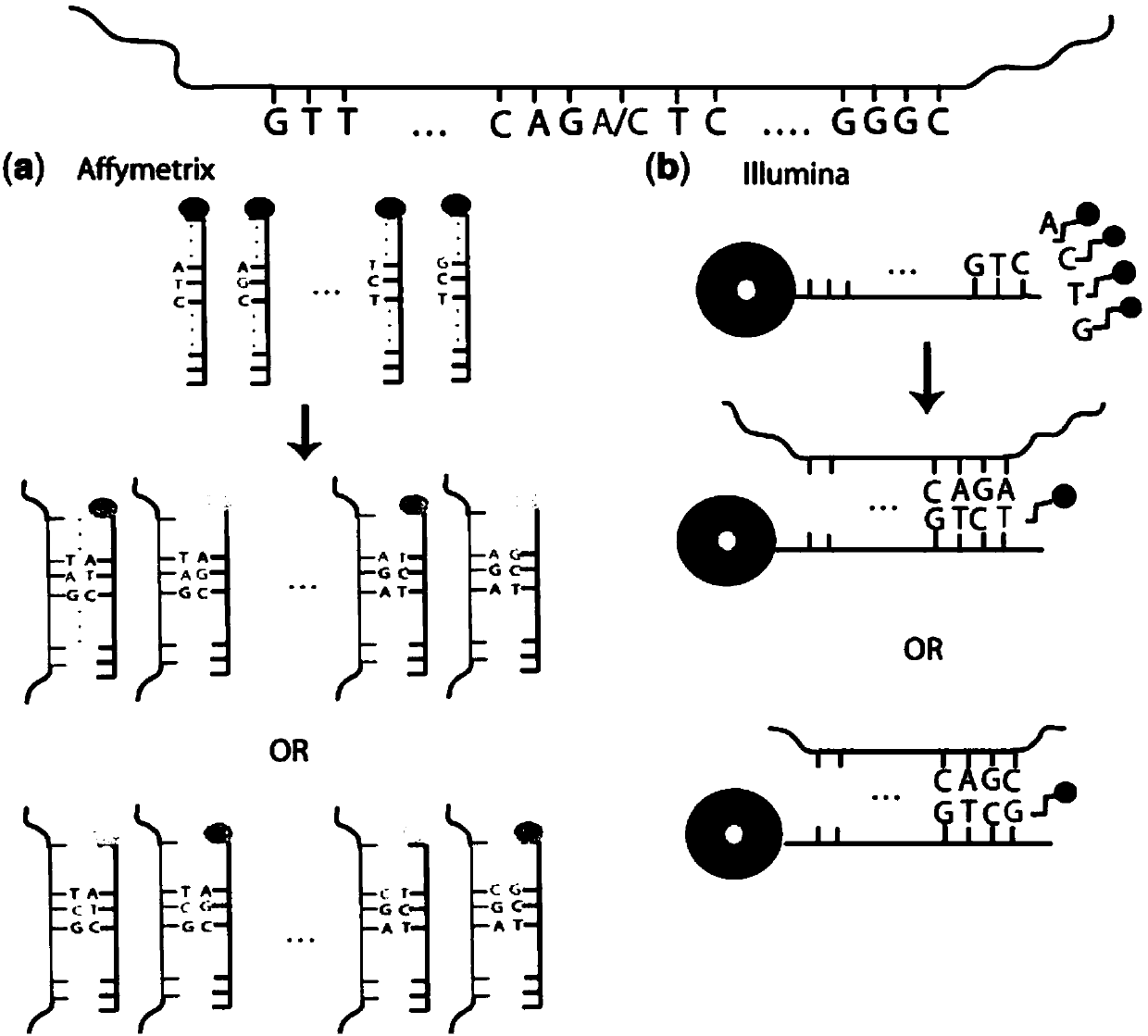
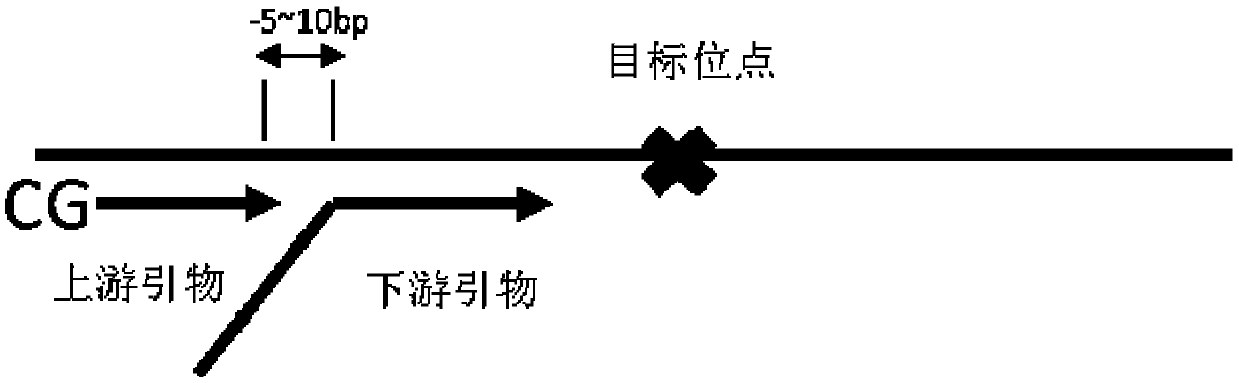
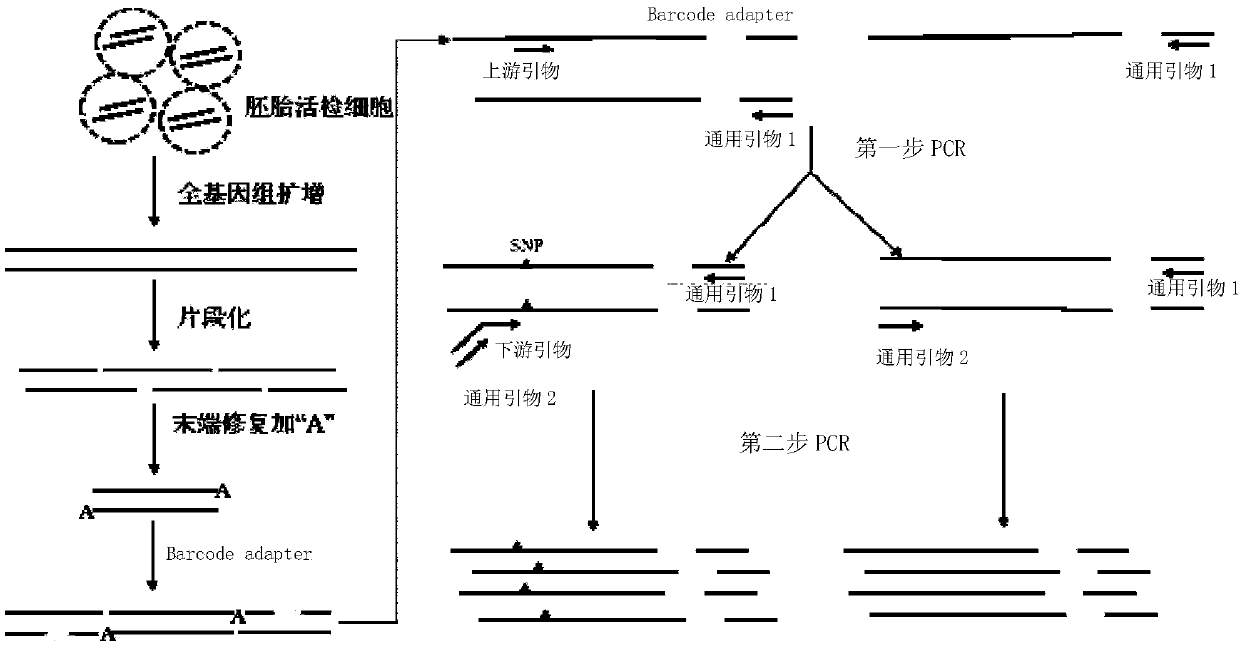
Genetic abnormality screening method for embryos
An embryo screening technology, applied in biochemical equipment and methods, microbiological measurement/testing, chemical library, etc., can solve problems such as complicated operation, abnormal number of chromosomes, inability to accurately detect copy number variation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Embodiment 1, primer design
[0094] β-thalassemia is mainly caused by mutations in the β-globin gene (HBB gene).
[0095] 20 sets of primers were designed, and the amplified sequences of the 20 sets of primers covered the entire HBB gene (ie, the part from the start codon to the stop codon in the genome). The sequences of the 20 sets of primers are shown in Table 1. HBB-USP-01 and HBB-DSP-01 form specific primer set 1, HBB-USP-02 and HBB-DSP-02 form specific primer set 2, and so on, ..., HBB-USP-20 and HBB-DSP- 20 constitute the specific primer set. The specific primer set was used to obtain all the SNP information in the HBB gene, and the SNP sites related to β-thalassemia were named as the pathogenic SNP sites.
[0096] Table 1
[0097]
[0098] In Table 1, all USP primers are upstream primers, and all DSP primers are downstream primers.
[0099] Randomly select 78 SNP sites with a minimum allele frequency (MAF) greater than 0.35 between the 2M region upstrea...
Embodiment 2
[0106] Example 2. Preimplantation chromosomal abnormality screening and β-thalassemia detection
[0107] Embryo samples: 9 pre-implantation embryos in IVF (E1 to E9; embryos at the blastomere stage, in practice embryos can also be at the blastocyst stage) from the same father and mother. Perform steps 2-11 individually for each embryo sample.
[0108] Human blood samples: whole blood of the father, whole blood of the mother, whole blood of the proband in the family (the proband here is the first affected child of the same father and mother). Perform Step 1 and Steps 3-11 individually for each human blood sample.
[0109] For the workflow diagram of steps 1-11, see image 3 .
[0110]1. Take 5ml of human blood sample, use the QIAGEN DNeasy Blood&Tissue kit and operate according to the instructions to obtain genomic DNA.
[0111] 2. Take a single cell from a blastomere stage embryo sample, and use the QIAGEN REPLI-g Single Cell Kit to operate according to the instructions to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com