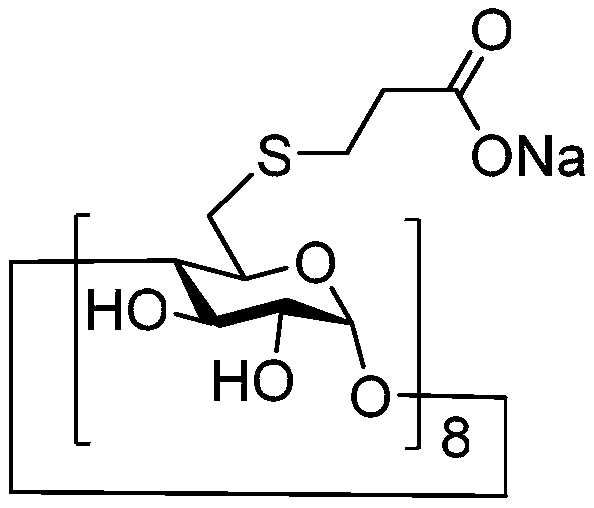
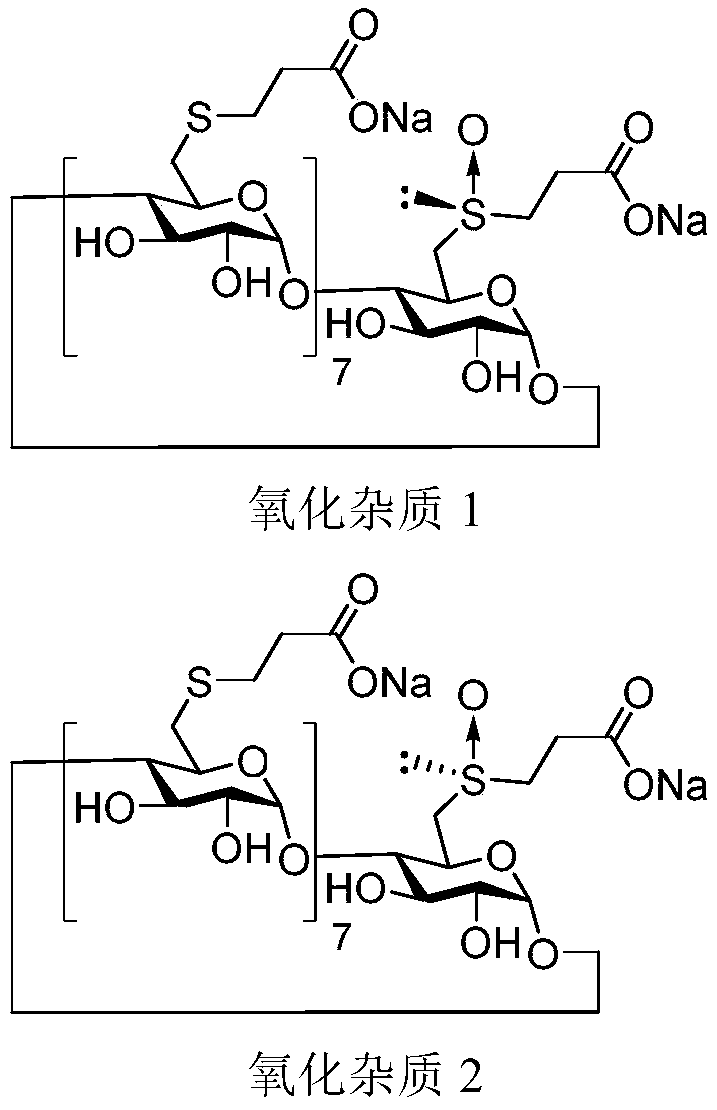
Stable Shugeng glucose sodium injection and preparation method thereof
A technology of sugammadex sodium and injection, which is applied in the field of medicine, can solve the problems such as the increase of impurities in sugammadex sodium injection and the color change of the liquid medicine, so as to achieve excellent product quality and stability, reduce the change of color, The effect of reducing the growth of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
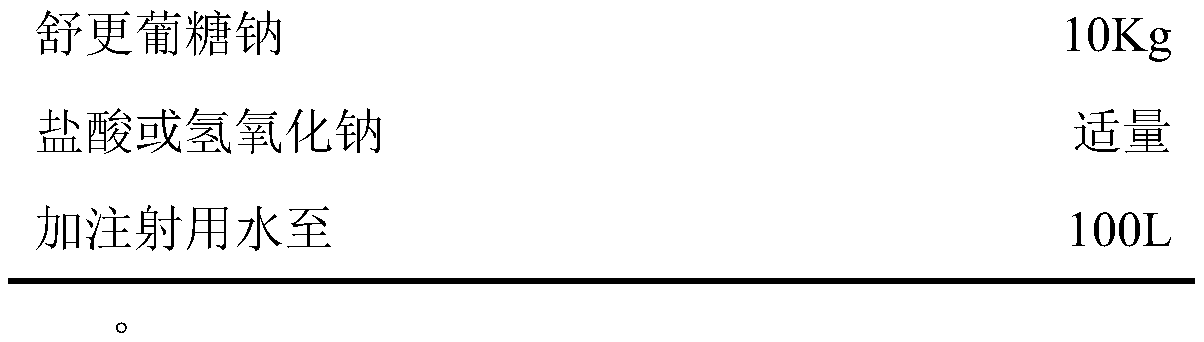
[0065] prescription
[0066] Sugammadex sodium injection (2ml: 200mg) production process prescription
[0067]
[0068] Preparation Process
[0069] 1. Preparation
[0070] 1) Turn on the vacuum of the batching tank to make the vacuum in the tank reach -0.085Mpa~-0.09Mpa, and fill it with 99.999% clean nitrogen gas to normal pressure;
[0071] 2) Add 80L of prescribed amount of water for injection into the liquid mixing tank, turn on the liquid mixing tank to stir, open the chilled water jacket of the liquid mixing tank, and lower the temperature of the water for injection to about 25°C;
[0072] 3) Bubble 99.999% clean nitrogen into the liquid mixing tank, monitor the dissolved oxygen in the water for injection online, so that the dissolved oxygen in the water for injection does not exceed 0.1ppm;
[0073] 4) When the dissolved oxygen in water for injection reaches below 0.1ppm, stop stirring in the liquid mixing tank, open the feeding port, add the prescribed amount of...
Embodiment 2
[0090] prescription
[0091] Sugammadex sodium injection (5ml: 500mg) production process prescription
[0092]
[0093] Preparation Process
[0094] 1. Preparation
[0095] 1) Turn on the vacuum of the batching tank to make the vacuum in the tank reach -0.085Mpa~-0.09Mpa, and fill it with 99.999% clean nitrogen gas to normal pressure;
[0096] 2) Add 80L of prescribed amount of water for injection into the liquid mixing tank, turn on the liquid mixing tank to stir, open the chilled water jacket of the liquid mixing tank, and lower the temperature of the water for injection to about 25°C;
[0097] 3) Bubble 99.999% clean nitrogen into the liquid mixing tank, monitor the dissolved oxygen in the water for injection online, so that the dissolved oxygen in the water for injection does not exceed 0.1ppm;
[0098] 4) When the dissolved oxygen in water for injection reaches below 0.1ppm, stop stirring in the liquid mixing tank, open the feeding port, add the prescribed amount of...
Embodiment 3
[0115] prescription
[0116] Sugammadex sodium injection (2ml: 200mg) production process prescription
[0117]
[0118] Preparation Process
[0119] 1. Preparation
[0120] 1) Turn on the vacuum of the batching tank to make the vacuum in the tank reach -0.085Mpa~-0.09Mpa, and fill it with 99.999% clean nitrogen gas to normal pressure;
[0121] 2) Add 80L of prescribed amount of water for injection into the liquid mixing tank, turn on the liquid mixing tank to stir, open the chilled water jacket of the liquid mixing tank, and lower the temperature of the water for injection to about 25°C;
[0122] 3) Bubble 99.999% clean nitrogen into the liquid mixing tank, monitor the dissolved oxygen in the water for injection online, so that the dissolved oxygen in the water for injection does not exceed 0.1ppm;
[0123] 4) When the dissolved oxygen in water for injection reaches below 0.1ppm, stop stirring in the liquid mixing tank, open the feeding port, add the prescribed amount of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com