Long-chain azobenzene compound, preparation method and applications thereof
A technology for azobenzene and compounds, which is applied in the field of long-chain azobenzene compounds and their preparation, can solve the problems of short recovery half-life and limited practical application, and achieve the effects of long recovery half-life, simple preparation and great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
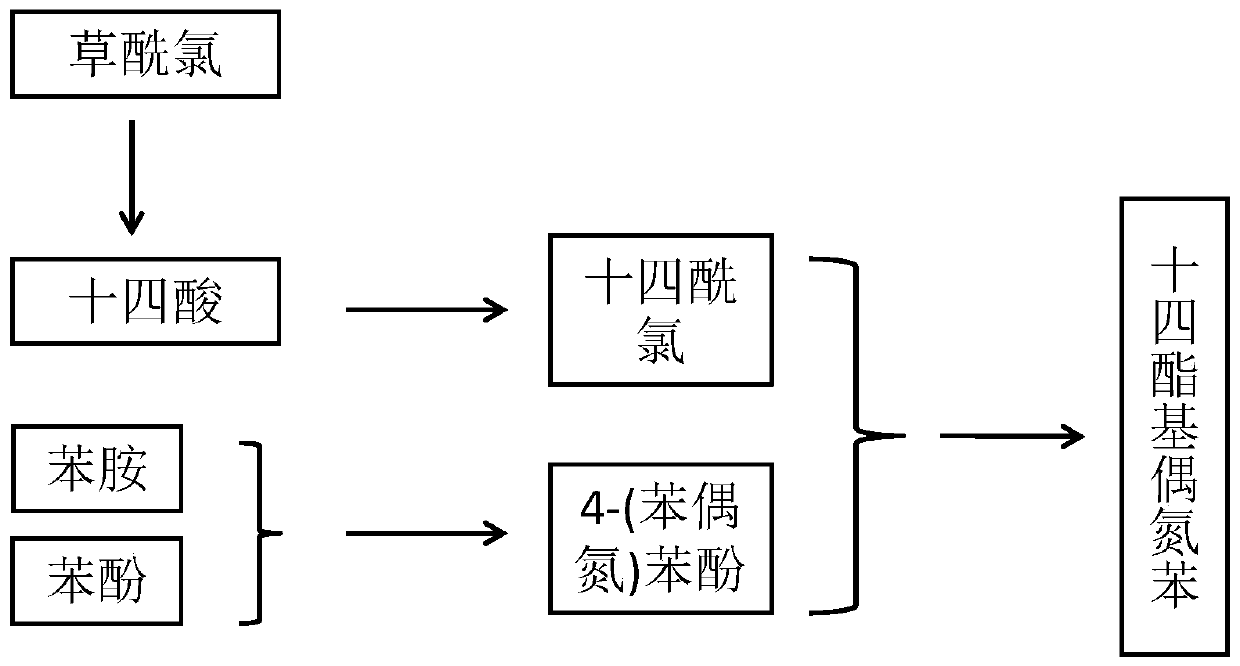
[0036] (1) Preparation of azobenzene derivatives: diazonium reaction of aminobenzene to generate diazonium salt, then coupling reaction with phenol, and filtration and recrystallization to obtain 4-(phenylazo)phenol.
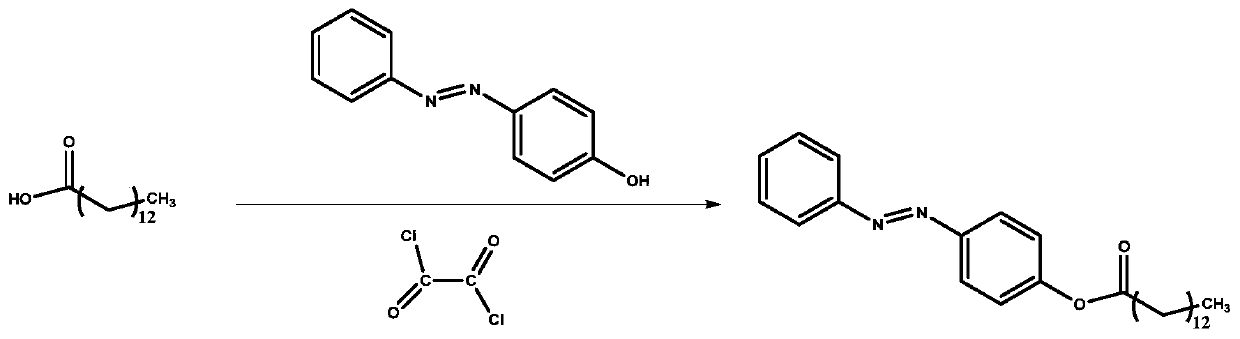
[0037] (2) 0.457g (2mmol) myristic acid was dissolved in 5mL dichloromethane, 0.256mL oxalyl chloride (3mmol) was added dropwise at room temperature and stirred for 10min. A drop of N,N-dimethylformamide was added to the mixture, the solution was stirred for 3 h, and dried to obtain myristyl chloride.
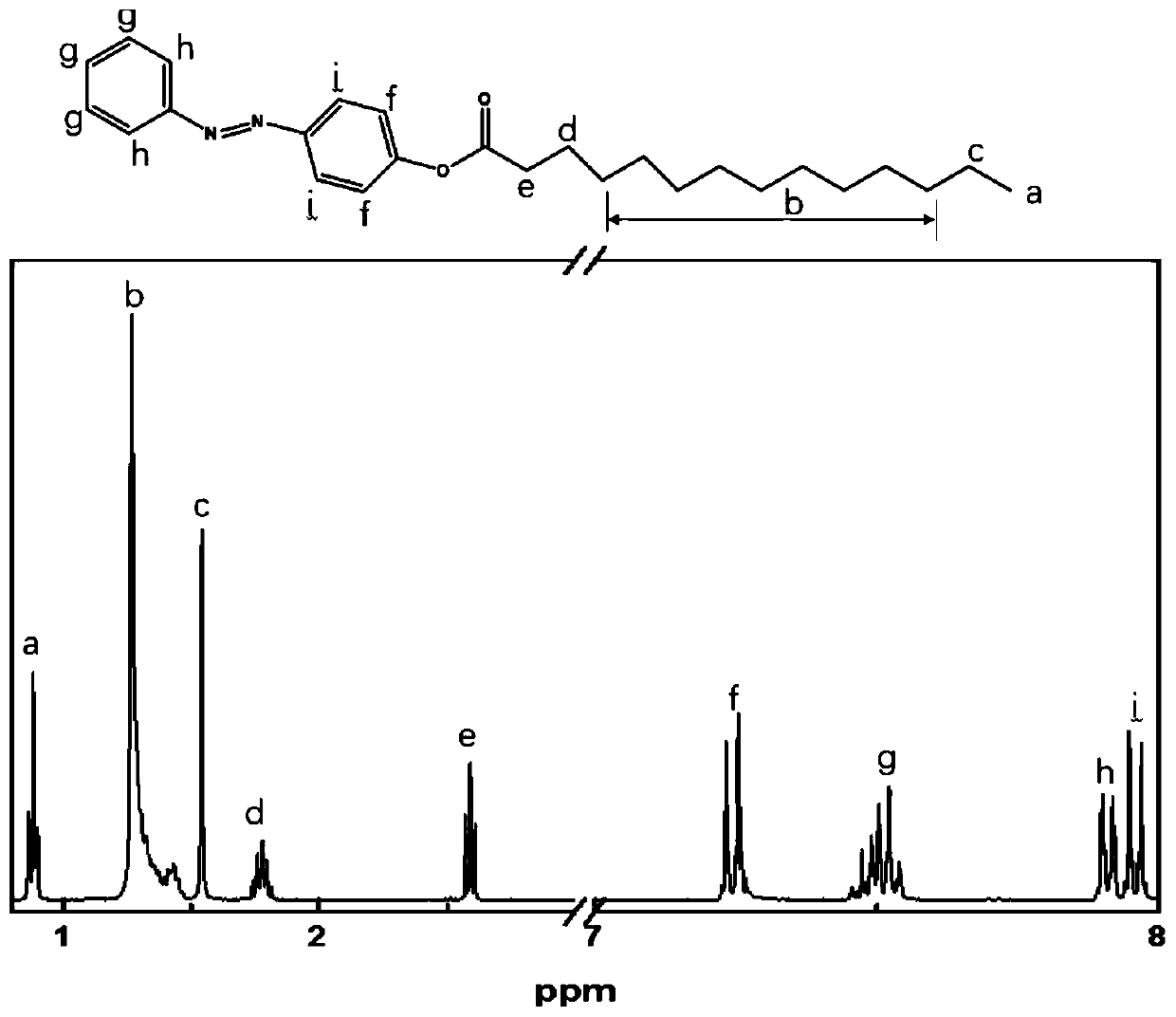
[0038] (3) Dissolve 0.494g tetradecanoyl chloride (2mmol) in 5mL dichloromethane, then add dropwise to 40mL dichloromethane containing 0.595g 4-(phenylazo)phenol (3mmol), 1.1mL (8mmol) triethylamine In methane solution, the mixture was stirred for 12h. The evaporation volume was reduced to 5 mL, and 20 mL of methanol was added to the reaction mixture to obtain a yellow precipitate, which was filtered and washed with methanol to obtain a long-chain azobenzene com...
Embodiment 2
[0042] (1) Preparation of azobenzene derivatives: diazonium reaction of aminobenzene to generate diazonium salt, then coupling reaction with phenol, and filtration and recrystallization to obtain 4-(phenylazo)phenol.
[0043] (2) 0.457g (2mmol) myristic acid was dissolved in 5mL dichloromethane, 0.256mL oxalyl chloride (3mmol) was added dropwise at room temperature and stirred for 10min. A drop of N,N-dimethylformamide was added to the mixture, the solution was stirred for 3 h, and dried to obtain myristyl chloride.
[0044] (3) Dissolve 0.494g myristyl chloride (2mmol) in 5mL dichloromethane, then add dropwise to 40mL dichloromethane containing 0.595g 4-(phenylazo)phenol (3mmol), 1.4mL (10mmol) triethylamine In methane solution, the mixture was stirred for 12h. The evaporation volume was reduced to 5 mL, and 20 mL of methanol was added to the reaction mixture to obtain a yellow precipitate, which was filtered and washed with methanol to obtain a long-chain azobenzene compoun...
Embodiment 3
[0046] (1) Preparation of azobenzene derivatives: diazonium reaction of aminobenzene to generate diazonium salt, then coupling reaction with phenol, and filtration and recrystallization to obtain 4-(phenylazo)phenol.
[0047] (2) 0.457g (2mmol) myristic acid was dissolved in 5mL dichloromethane, 0.218mL thionyl chloride (3mmol) was added dropwise at room temperature and stirred for 10min. A drop of N,N-dimethylformamide was added to the mixture, the solution was stirred for 3 h, and dried to obtain myristyl chloride.
[0048] (3) Dissolve 0.494g tetradecanoyl chloride (2mmol) in 5mL dichloromethane, then add dropwise to 40mL dichloromethane containing 0.595g 4-(phenylazo)phenol (3mmol), 1.1mL (8mmol) triethylamine In methane solution, the mixture was stirred for 12h. The evaporation volume was reduced to 5 mL, and 20 mL of methanol was added to the reaction mixture to obtain a yellow precipitate, which was filtered and washed with methanol to obtain a long-chain azobenzene co...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap