Application of recombinant mesenchymal stem cells in preparing drugs for treating myocardial infarction
A technology of stem cells and recombinant plasmids, applied in bone/connective tissue cells, cells modified by introducing foreign genetic material, applications, etc., can solve problems such as application, low effect and scar tissue formation that are not involved in the treatment of cardiac infarction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1. Isolation of rat mesenchymal stem cells:
[0042] 1) Under sterile conditions, separate the femur and tibia of the rat, and flush the bone marrow in the marrow cavity with a 1 mL syringe.
[0043] 2) The washed bone marrow was filtered through a 70 μm filter, and centrifuged at 1350 rpm for 15 minutes.
[0044] 3) Add erythrocyte lysate to resuspend and lyse for 5 minutes to lyse the erythrocytes in the bone marrow.
[0045] 4) Wash 1-2 times with PBS, then resuspend with freshly prepared DMEM / F12+10% FBS and inoculate in culture dish.
[0046] 5) After 72 hours, the non-adherent cells were sucked off, and the medium was changed every 2-3 days thereafter, and the cell density in the culture dish reached 80% to be subcultured. At this time, the cells were marked as P0 generation.
Embodiment 2
[0047] Example 2. Identification of rat mesenchymal stem cells:
[0048] 1) Take the P3-P5 generation cells in good growth state, digest the cells with 0.25% trypsin+EDTA, collect the cells and count them.
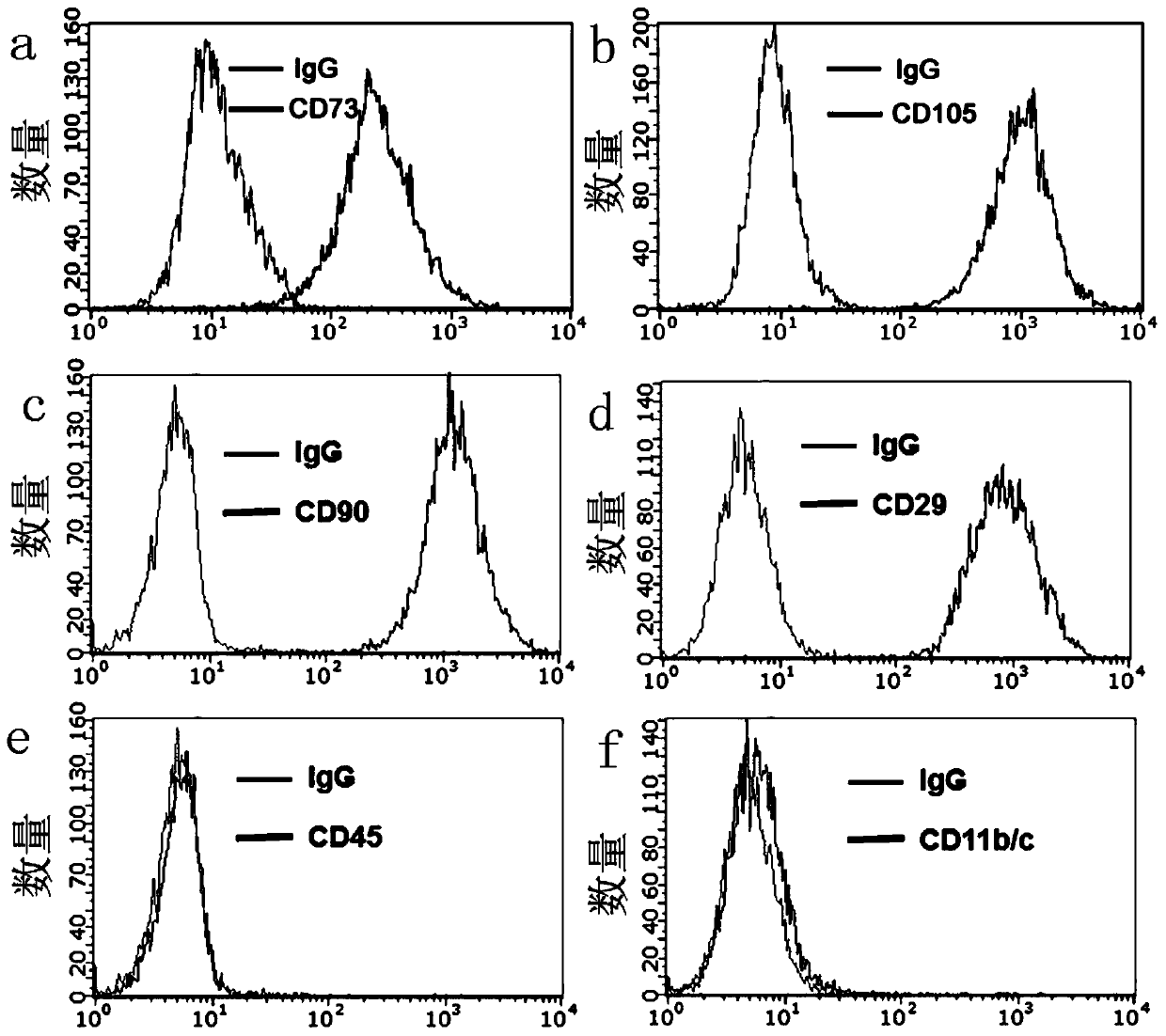
[0049] 2) Put 2×10 5 The cells were divided into a portion, and each portion was added with CD105-PE (12-1051, eBioscience), CD73-PE (551123, BD), CD90-PE (551401, BD), CD29-FITC (555005, BD), CD45- PE (6828, BD) and CD11b / c-FITC (b141556, Biolegend) antibodies.
[0050] 3) After mixing, incubate at 4°C in the dark for 20 minutes, wash with PBS twice, and then detect with a Guavaeasycyte flow cytometer. Test results such as figure 1 , the abscissa indicates the fluorescence intensity, and the ordinate indicates the number of cells.
Embodiment 3
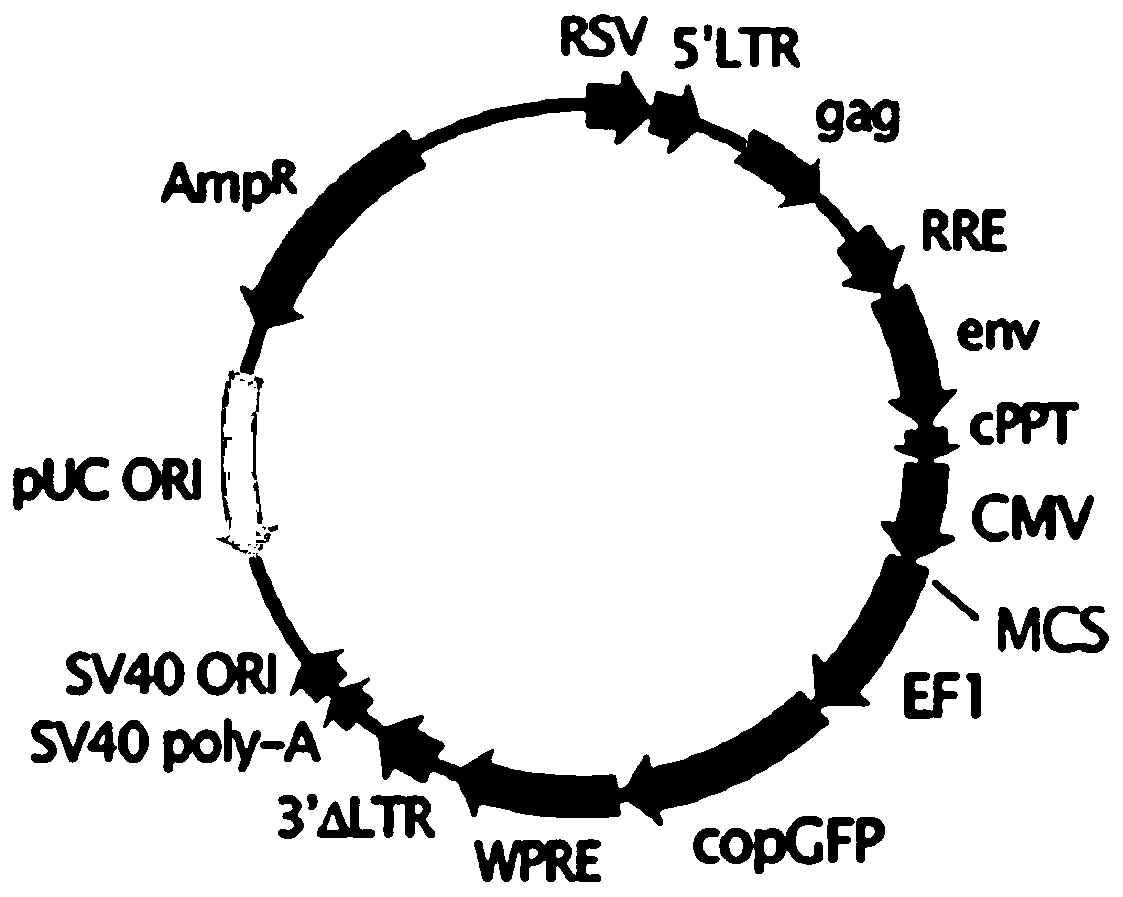
[0051] Example 3. Design of IL-33 high expression plasmid for editing mesenchymal stem cells:
[0052] 1) Find the sequence of rat IL-33 on the NCBI website, and find that the gene has only one transcript, and the CDS region is continuous in the mRNA. The nucleotide sequence of IL-33 CDS is shown in SEQ ID No.3.
[0053] 2) It is predicted on the primer5 software that there are no restriction endonucleases BamHI and XbaI in the CDS region, so the primers in this region are designed to add BamHI and XbaI endonuclease sequences at both ends.
[0054] Primer sequence:
[0055] Front primer sequence: 5'TTAAGGATCCGCCACCATGAGACCTAGAATGAAGTATTCGAAC3' (SEQ ID No.1);
[0056] Rear primer sequence: 5'TTTTTCTAGATTACATCTTAGAGAGCTTAAACATGATAT3' (SEQ ID No. 2).
[0057] Using the front and back primer sequences, the complete sequence of IL-33 was obtained by PCR amplification. The PCR amplification conditions were as follows: 95°C, 5min; (95°C, 30s-45s; 60°C, 45s; 72°C, 45s-60s) -35-40 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com