Composition with skin whitening effect, skin-whitening soluble micro-needle patch with high drug loading capacity, and preparation method of skin-whitening soluble micro-needle patch
A high drug-loading, soluble technology, applied in the field of microneedles, can solve the problems that microneedles cannot pierce the skin, low microneedle strength, and low drug loading, so as to reduce skin pigmentation, resist ultraviolet rays, and achieve excellent whitening skin effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
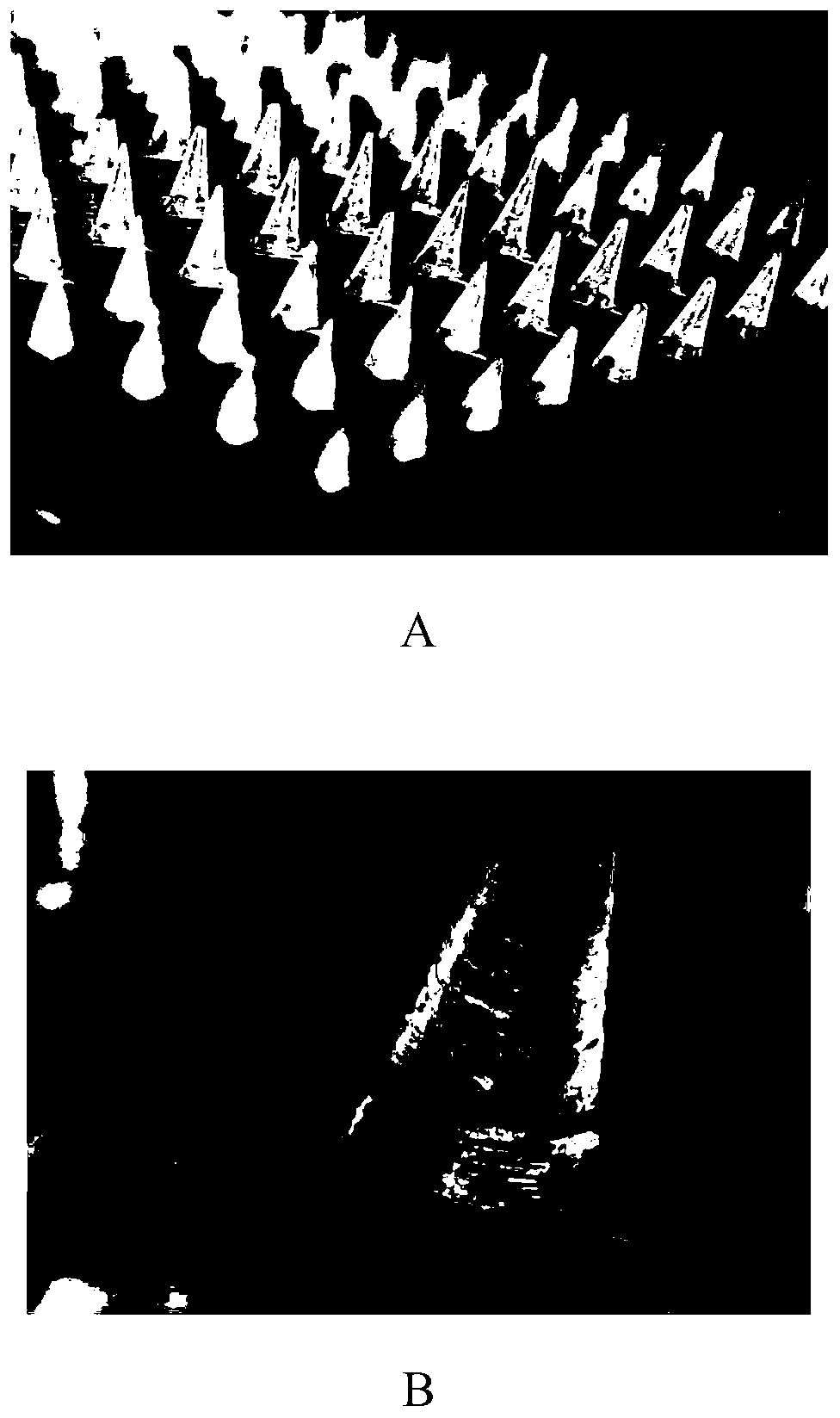
[0061] The whitening soluble microneedle patch with high drug loading provided in this embodiment is composed of a needle point and a base; the raw materials of the needle point are composed as follows (in parts by weight): 13 parts of arbutin, 15 parts of magnesium ascorbyl phosphate, 7 parts of adenosine, 0.4 parts of sturgeon caviar extract, 106.2 parts of sodium hyaluronate (molecular weight of 280kDa), 6 parts of polyvinylpyrrolidone (molecular weight of 1300kDa), 12 parts of glycerin, 6 parts of hydroxypropyl cellulose; The composition of raw materials is as follows: 1.1 parts of sodium hyaluronate (molecular weight: 280Kda), 0.1 parts of glycerin, and 0.1 parts of hydroxypropyl cellulose.
[0062] The preparation method of the whitening soluble microneedle patch with high drug loading provided in this example is as follows:
[0063] S1: Preparation of needle tips: After uniformly mixing the above-mentioned raw materials for preparing needle tips, add 591.4 parts of dist...
Embodiment 2
[0066] The whitening soluble microneedle patch with high drug loading provided in this embodiment is composed of a needle point and a base; the raw materials of the needle point are composed as follows (in parts by weight): 13 parts of arbutin, 15 parts of magnesium ascorbyl phosphate, 7 parts of adenosine, 0.4 parts of sturgeon caviar extract, 70.8 parts of hyaluronic acid (molecular weight of 280kDa), 6 parts of polyvinylpyrrolidone (molecular weight of 1300kDa), 12 parts of glycerin, 6 parts of hydroxypropyl cellulose; The composition of the raw materials is as follows: 1.1 parts of sodium hyaluronate (molecular weight: 280 kDa), 0.1 parts of glycerin, and 0.1 parts of hydroxypropyl cellulose.
[0067] The preparation method of the whitening soluble microneedle patch with high drug loading provided in this example is as follows:
[0068] S1: Preparation of needle tips: After uniformly mixing the above-mentioned raw materials for preparing needle tips, add 591.4 parts of dis...
Embodiment 3
[0071] The whitening soluble microneedle patch with high drug loading provided in this embodiment is composed of a needle point and a base; the raw materials of the needle point are composed as follows (in parts by weight): 13 parts of arbutin, 15 parts of magnesium ascorbyl phosphate, 7 parts of adenosine, 0.4 parts of sturgeon caviar extract, 35.4 parts of hyaluronic acid (molecular weight: 280kDa), 6 parts of polyvinylpyrrolidone (molecular weight: 1300kDa), 12 parts of glycerin, 6 parts of hydroxypropyl cellulose; the raw materials of the base The composition is as follows: 1.1 parts of sodium hyaluronate (molecular weight: 280 kDa), 0.1 parts of glycerin, and 0.1 parts of hydroxypropyl cellulose.
[0072] The preparation method of the whitening soluble microneedle patch with high drug loading provided in this example is as follows:
[0073] S1: Preparation of needle tips: After uniformly mixing the above-mentioned raw materials for preparing needle tips, add 591.4 parts o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com