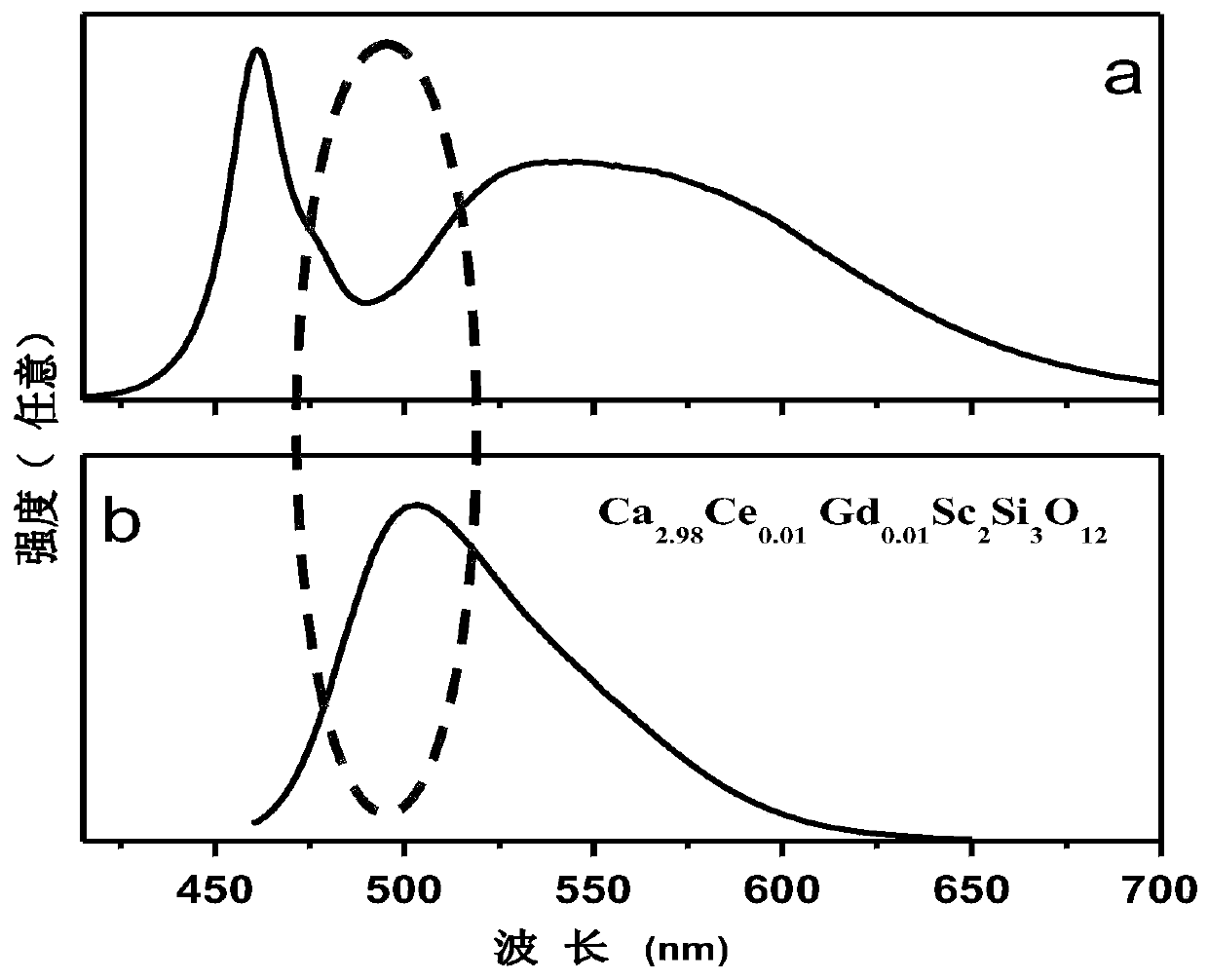
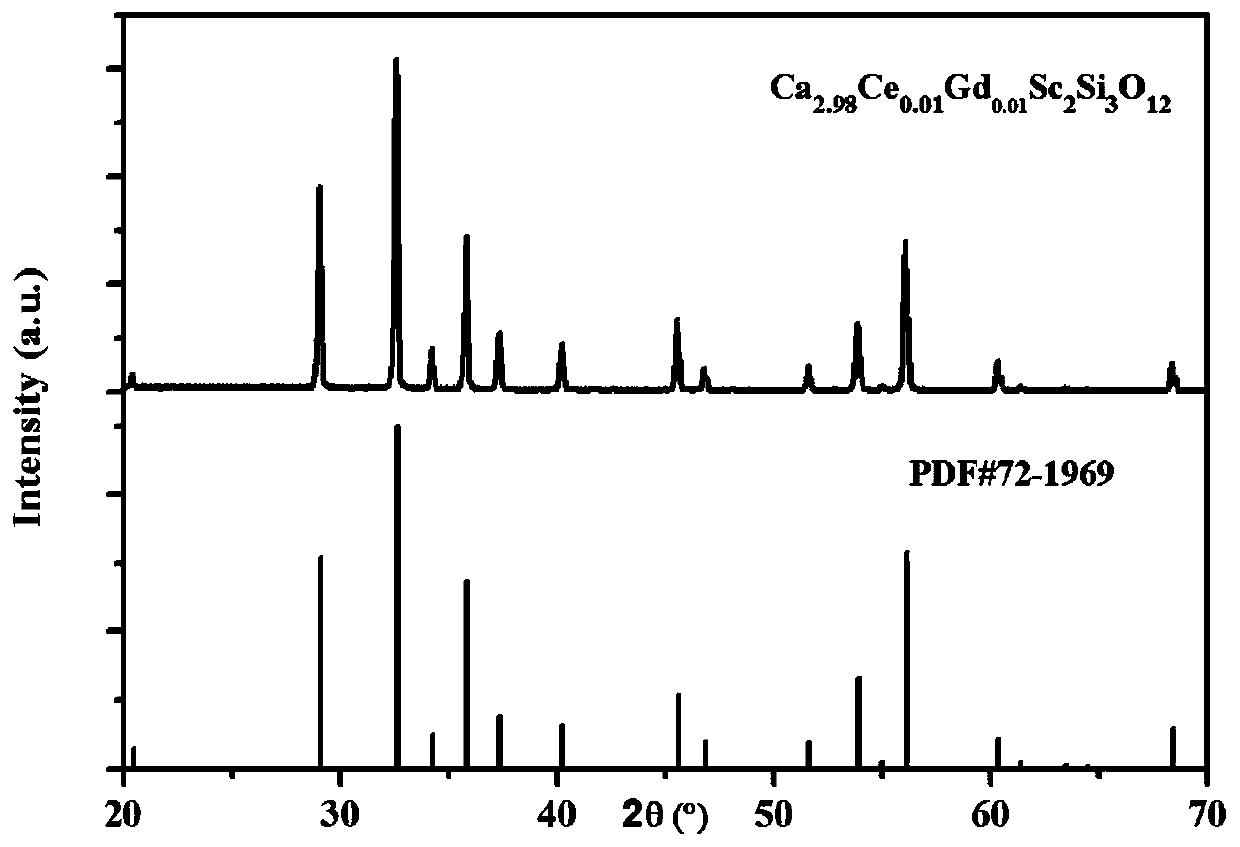
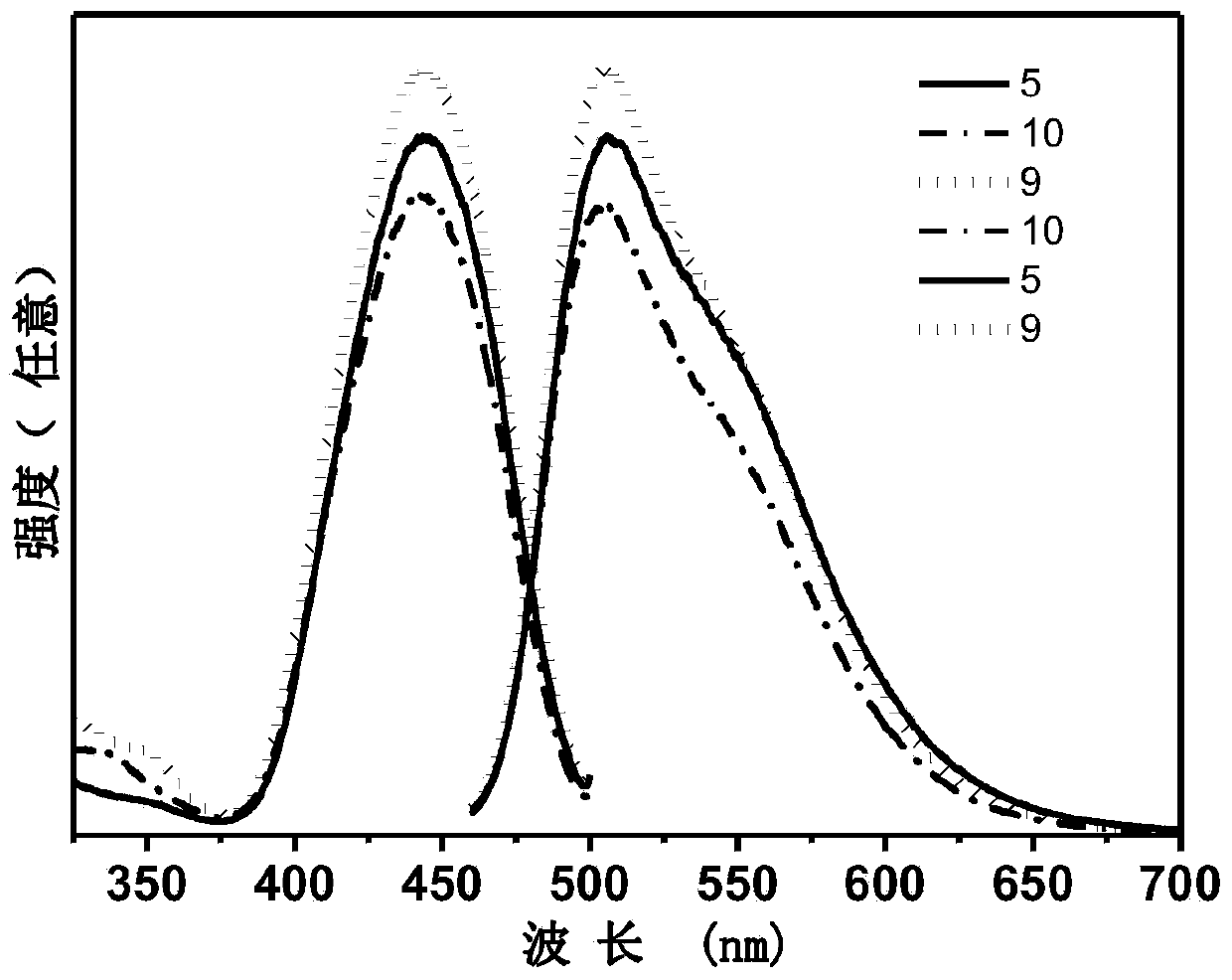
Blue-green light emitting scandium silicate fluorescent ceramic and preparation method thereof
A scandium silicate and fluorescent ceramic technology is applied in the field of scandium silicate fluorescent ceramics and their preparation, which can solve the problems of low luminous efficiency, low transmittance, low density of fluorescent ceramics, etc. The effect of good stability, quantum efficiency and excellent temperature characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Ca 2.9395 Ce 0.0005 Y 0.06 sc 2 Si 3 o 12 preparation of
[0049] Weigh Ca(NO 3 ) 2 .4H 2 O: 34.7081 grams, Ce(NO 3 ) 3 .6H 2 O: 0.0109 grams, Y (NO 3 ) 3 .6H 2 O: 1.1492 g, Sc(NO 3 ) 3 : 23.097 grams, the above-mentioned materials are dissolved in deionized water, and fully stirred until completely dissolved; measure TEOS: 33.5ml is dissolved in absolute ethanol, then, the above-mentioned two kinds of solutions are mixed and fully stirred to obtain a mixed solution;
[0050] Then the obtained mixed solution was slowly dried at 60° C. until a transparent gel was obtained, and further dried at 110° C. to obtain a dry gel.
[0051] Put the obtained xerogel into a high-temperature furnace and anneal at 600° C. for 5 hours to remove acid groups and organic matter, thereby obtaining a loose precursor powder.
[0052] Subsequently, the precursor powder was firstly dry-pressed in an axial unidirectional pressure mold with a pressure of 16MPa and a dwell time o...
Embodiment 2
[0057] Ca 2.995 Ce 0.005 sc 1.94 Zr 0.05 f 0.01 Si 3 o 12 preparation of
[0058] Weigh Ca(NO 3 ) 2 .4H 2 O: 35.3635 grams, Ce(NO 3 ) 3 .6H 2 O: 0.1085, Sc(NO 3 ) 3 : 22.4041 g, Zr(NO 3 ) 4 .5H 2 O: 1.0733 g, HfCl 4 : 0.1602g, urea: 15.015g, TEOS: 31.2495g. Dissolve the above materials in deionized water and stir until completely dissolved; measure 33.5ml of TEOS and dissolve in absolute ethanol. Then, the above two solutions are mixed and fully stirred to obtain a mixed solution;
[0059] The resulting mixed solution was dried slowly at 70°C until a transparent gel was obtained, and further dried at 90°C to obtain a xerogel.
[0060] Put the obtained xerogel into a high-temperature furnace and anneal at 800° C. for 8 hours to remove acid groups and organic matter, thereby obtaining a loose precursor powder.
[0061] The sieved precursor powder was first dry-pressed in an axial unidirectional pressure mold, and then pressed into a green body by cold isostati...
Embodiment 3
[0066] Ca 2.99 Ce 0.01 sc 2 Si 2.94 Al 0.06 o 12 preparation of
[0067] Weigh Ca(NO 3 ) 2 .4H 2 O: 35.3044 grams, Ce(NO 3 ) 3 .6H 2 O: 0.2171 g, Sc(NO 3 ) 3 : 23.097 grams, TEOS: 30.6245 grams, Al(NO 3 ) 3 .9H 2 O: 1.1254 grams, anhydrous citric acid: 48.0325g, dissolve each material in deionized water, and fully stir until completely dissolved; measure (TEOS: 32.8ml and dissolve in absolute ethanol. Then, the above two solutions Mix and stir thoroughly to obtain a mixed solution;
[0068] The resulting mixed solution was slowly dried at 80°C until a transparent gel was obtained, and further dried at 120°C to obtain a xerogel.
[0069] Put the obtained xerogel into a high-temperature furnace and anneal at 1000°C for 3 hours to remove acid groups and organic matter, thereby obtaining a loose precursor powder.
[0070] The sieved precursor powder is first dry-pressed in an axial one-way pressurized mold with a pressure of 20MPa and a holding time of 2min, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com