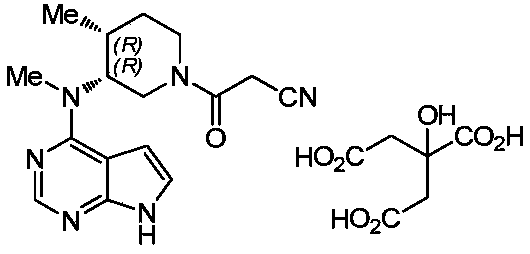
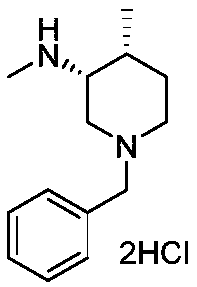
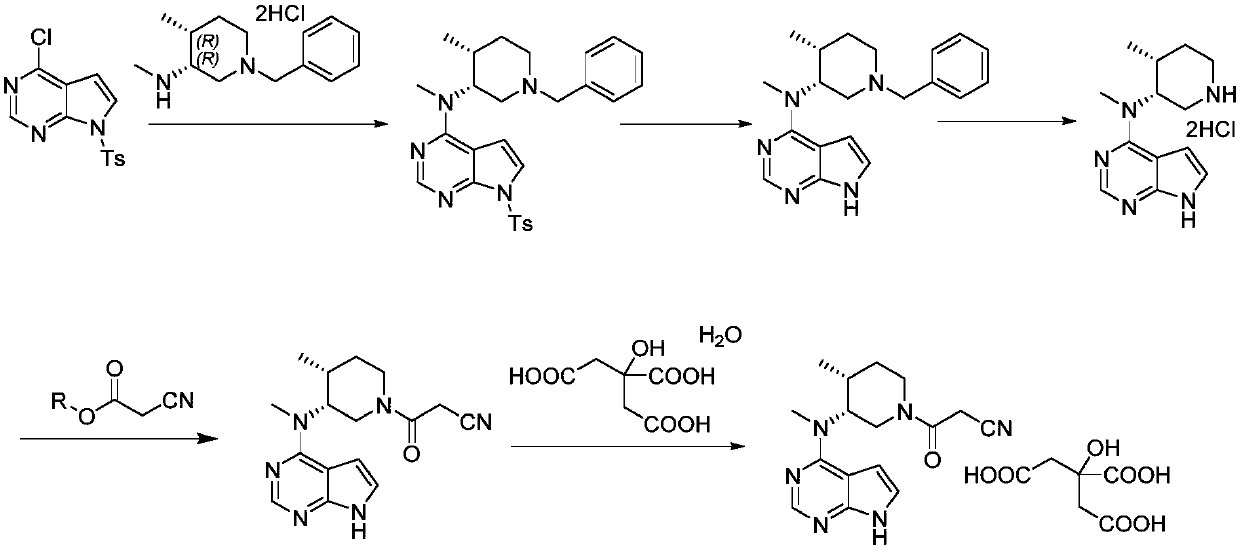
Preparation methods of tofacitinib citrate intermediate and tofacitinib citrate
A technology of tofacitinib and citric acid, which is applied in the preparation of tofacitinib citrate and the field of tofacitinib citrate intermediates, which can solve the problems of high catalyst consumption and catalyst poisoning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] A preparation method of tofacitinib citrate, comprising the following steps:
[0073] One, the preparation of tofacitinib citrate intermediate; The described tofacitinib citrate intermediate is (3R,4R)-1-benzyl-N,4-dimethylpiperidin-3-amine double hydrochloride;
[0074] 2. N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7-p-toluenesulfonyl-pyrrolo[2,3-d]pyrimidine -The preparation of 4-amine; Concretely comprise the following steps:
[0075] A. Mix 10.0kg purified water, 2.6kg acetonitrile, 1.7kg (3R,4R)-1-benzyl-N,4-dimethylpiperidin-3-amine dihydrochloride and 2.3kg potassium carbonate, stir to dissolve;
[0076] B. Add 1.8kg of 4-chloro-7-p-toluenesulfonyl-pyrrolo[2,3-d]pyrimidin-4-amine to the result of the previous step in several times, and raise the temperature to 75-85°C for 10 hours to carry out HPLC detection;
[0077] C. Cool down to 20-30°C and keep stirring for 2 hours, filter with suction, wash the filter cake with 3kg of purified water, and set...
Embodiment 2
[0095] On the basis of Example 1, the preparation method of the tofacitinib citrate intermediate (3R,4R)-1-benzyl-N,4-dimethylpiperidin-3-amine dihydrochloride Specifically:
[0096]
[0097] (1) Preparation of N-(1-benzyl-4-methyl-1,2,5,6-tetrahydropiperidin-3-yl)acetamide; specifically comprising the following steps:
[0098] A. Add 2.2kg of 3-amino-4-methyl-pyridine into 15kg of dichloromethane and 22kg of toluene, cool down to 0-10°C, add 1.7kg of acetyl chloride dropwise, and react at 20-25°C for 10 hours; Below ℃, add aqueous sodium hydroxide solution dropwise, adjust the pH to 8-9, separate the lower aqueous phase; concentrate the organic phase under reduced pressure to a volume of 23-25L;
[0099] B. Add 1.9 kg of benzyl chloride to the resultant of the previous step, heat up to 70-80°C for reaction, and separate the dichloromethane distilled during the heating process; cool down to 0-10°C after the reaction;
[0100] C. Control the temperature below 10°C, and add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com