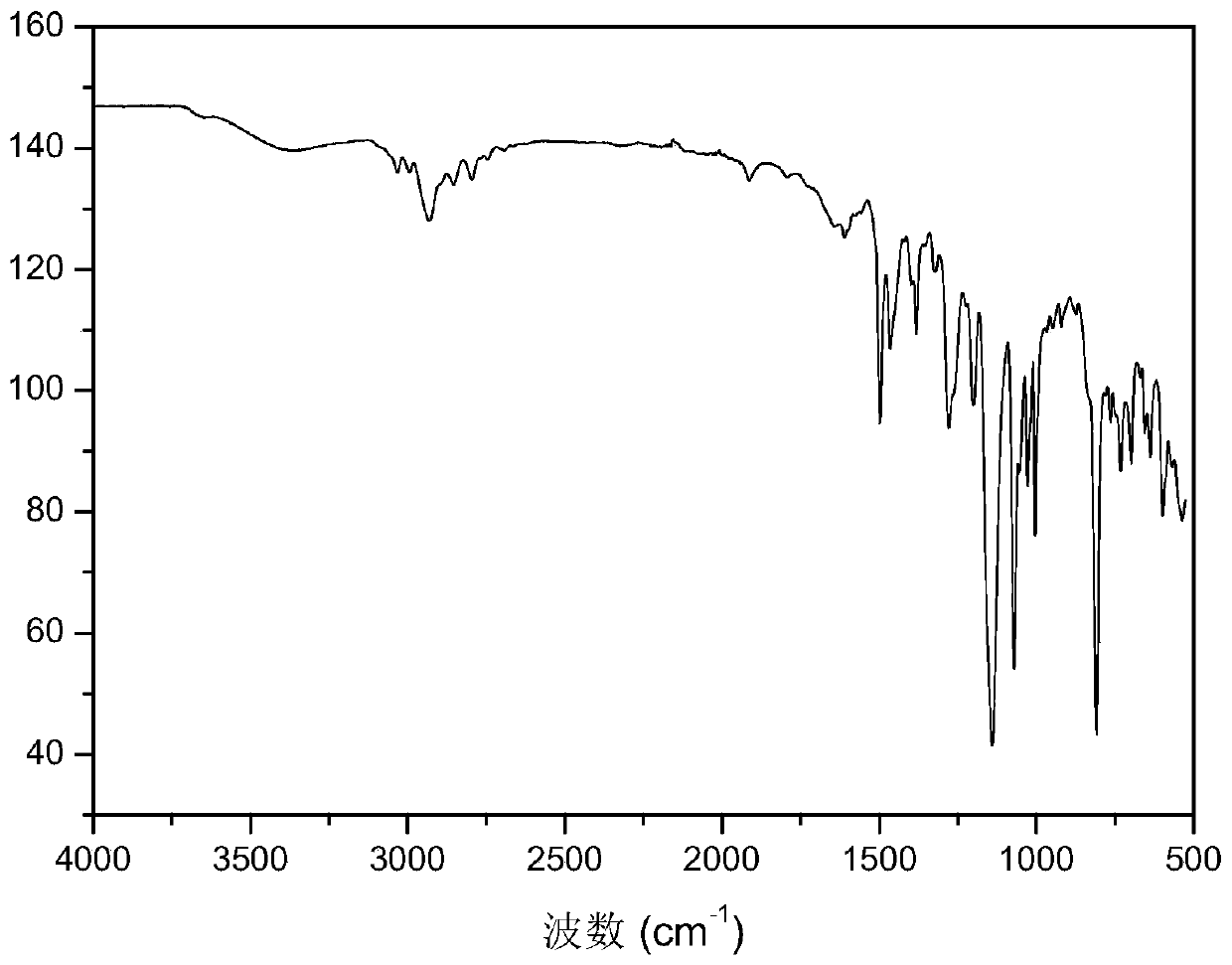
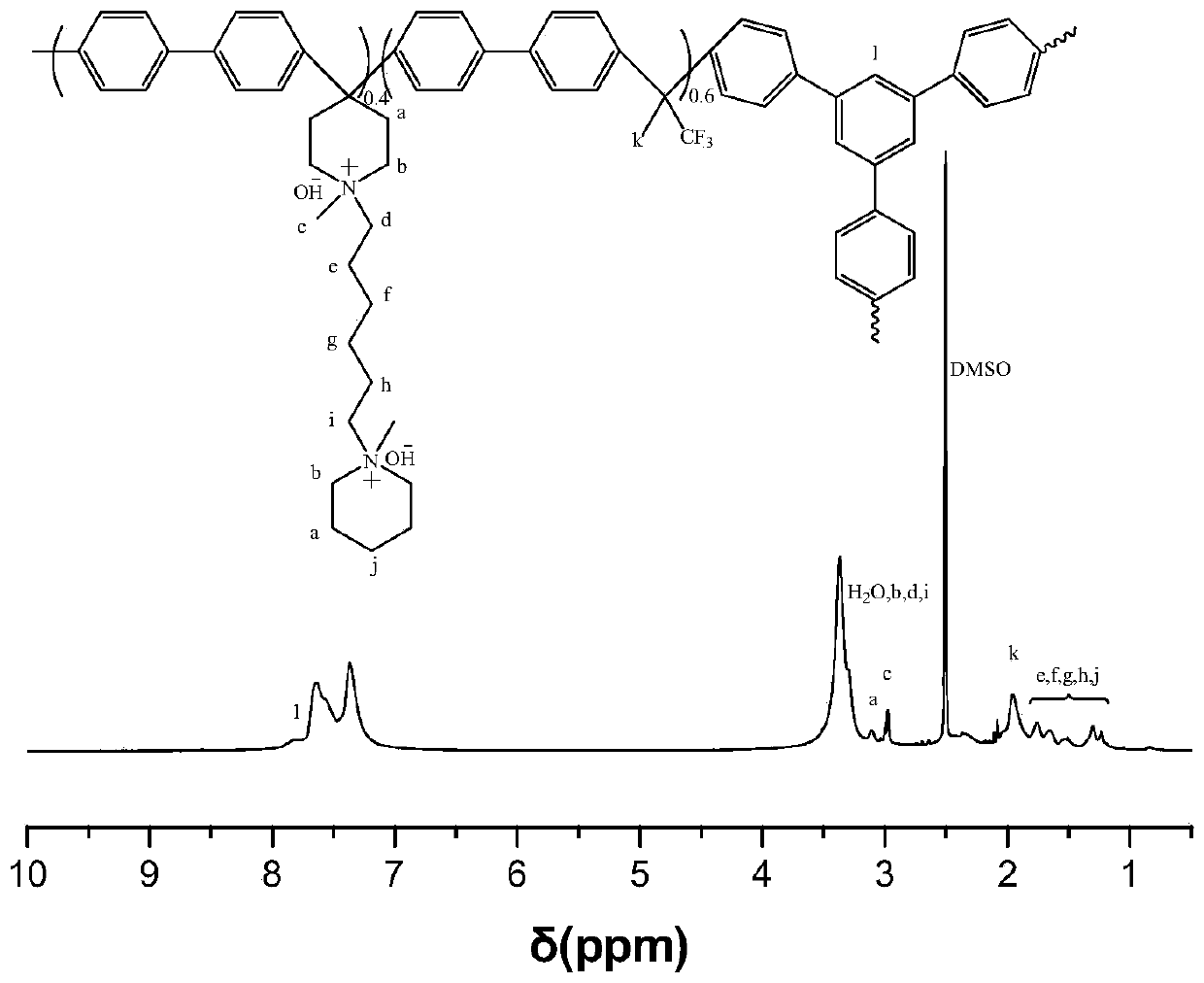
Method for preparing alkaline anion exchange membrane based on branched oxygen-free main chain
A basic anion and main chain technology, applied in the field of preparation of branched cationic alkaline anion membranes, can solve the problems of low electrical conductivity and poor chemical stability, and achieve the effect of improving electrical conductivity and enhancing alkali resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Prepare a polymer main chain with a branching degree of 1. Take 0.0199g of 1,3,5-triphenylbenzene and 0.9873g of biphenyl into a 25ml round bottom flask, then add 2.8ml of dichloromethane and stir at room temperature until completely dissolved, then add 0.41mL of N-methylpiperidone and 0.45 mL of trifluoroacetone, then put the round bottom flask into ice bath, then add 0.64mL of trifluoroacetic acid and 6.8mL of trifluoromethanesulfonic acid dropwise using a constant pressure funnel and stir for 5h, then pour the mixture into 1M NaOH solution for washing After 12 hours, it was washed with deionized water until neutral to obtain a solid product, and the obtained solid was dried in a vacuum oven at 50° C. for 48 hours.
[0033] (2) Preparation of ionizing reagents. Measure 24.28mL of 1,6-dibromohexane and 12.09mL of N-methylpiperidine into a 100mL round bottom flask, then add 40mL of ethyl acetate into the flask, stir at room temperature until completely dissolved, t...
Embodiment 2
[0039] (1) Prepare a polymer main chain with a branching degree of 3. Take 0.0597g of 1,3,5-triphenylbenzene and 0.9573g of biphenyl into a 25ml round bottom flask, add 2.8ml of dichloromethane and stir at room temperature until completely dissolved, then add 0.41mL of N-methylpiperidone and 0.45 mL of trifluoroacetone, then put the round-bottom flask into an ice bath, then add 0.64 mL of trifluoroacetic acid and 6.8 mL of trifluoromethanesulfonic acid dropwise using a constant pressure funnel, stir for 3 h, and then pour the mixture into 1M NaOH solution After washing for 12 hours, it was washed with deionized water until neutral to obtain a solid product, and the obtained solid was dried in a vacuum oven at 50° C. for 48 hours.
[0040] (2) Preparation of ionizing reagents. Measure 32.37mL of 1,6-dibromohexane and 12.09mL of N-methylpiperidine into a 100mL round bottom flask, then add 40mL of ethyl acetate into the flask, stir at room temperature until completely dissolve...
Embodiment 3
[0046] (1) Prepare a polymer main chain with a branching degree of 5. Take 0.0996g of 1,3,5-triphenylbenzene and 0.9272g of biphenyl into a 25ml round bottom flask, add 2.8ml of dichloromethane and stir at room temperature until completely dissolved, then add 0.41mL of N-methylpiperidone and 0.45 mL of trifluoroacetone, then put the round-bottomed flask in an ice bath, then add 0.64 mL of trifluoroacetic acid and 6.8 mL of trifluoromethanesulfonic acid dropwise using a constant pressure funnel, stir for 2 h, and then introduce the mixture into 1M NaOH solution for washing After 12 hours, it was washed with deionized water until neutral to obtain a solid product, and the obtained solid was dried in a vacuum oven at 50° C. for 48 hours.
[0047] (2) Preparation of ionizing reagents. Measure 80.92mL of 1,6-dibromohexane and 12.09mL of N-methylpiperidine into a 100mL round bottom flask, then add 120mL of ethyl acetate into the flask, stir at room temperature until completely diss...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com