Compounds and compositions for treating hematological disorders
A compound, leukemia technology, applied in drug combinations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of AML death and low survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
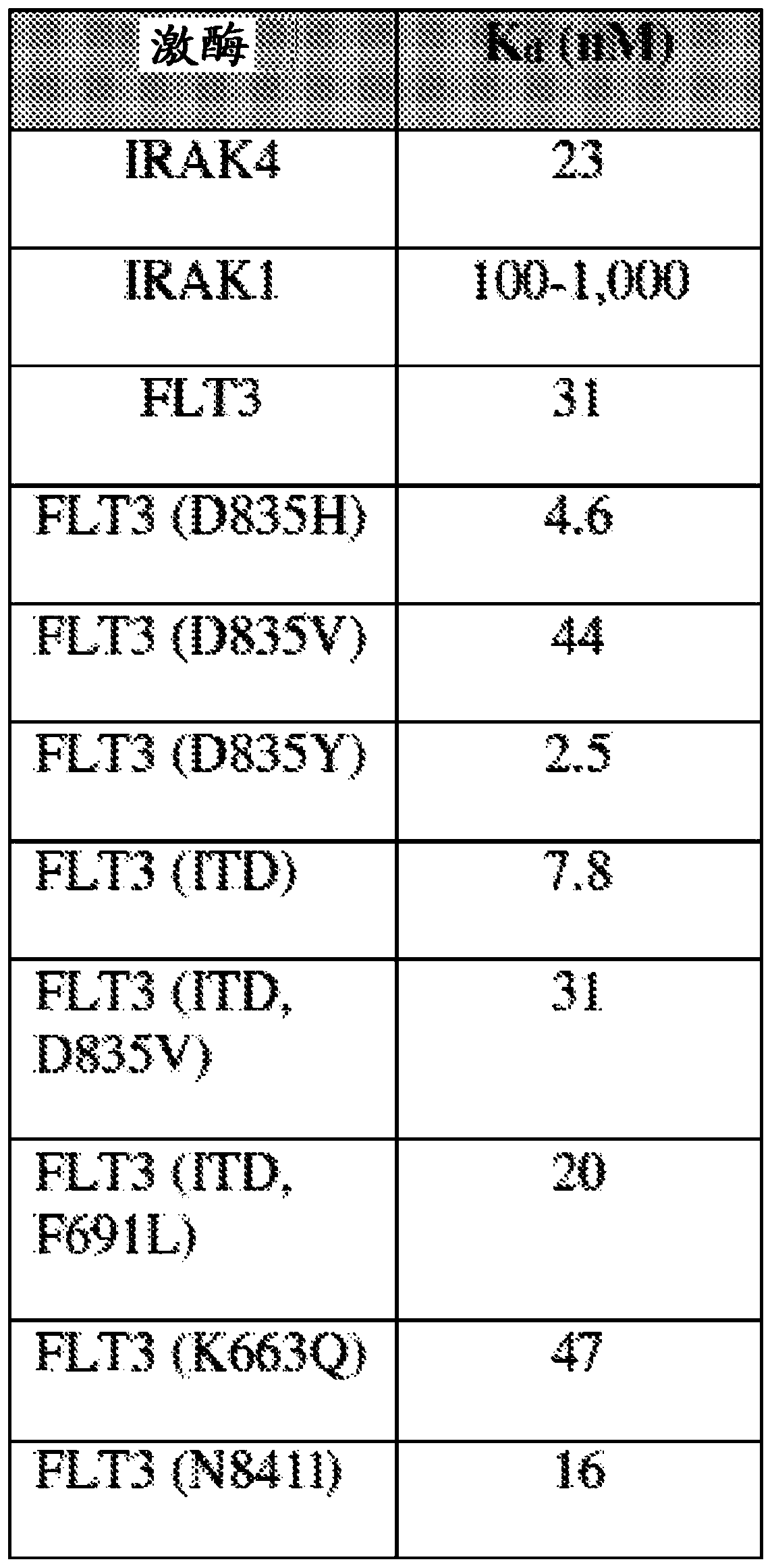
[0339] Example 1: Inhibition of FLT-3 by Compound A
[0340] Compounds were tested for inhibition of FLT-3 wild type using the substrate peptide EAIYAAPFAKKK. Flt3(h) (14-500, GenBank NM_004119) was incubated with 8 mM MOPS (pH 7.0), 0.2 mM EDTA, 50 μM EAYAAPFAKKK, 10 mM magnesium acetate and [γ-33P]-ATP (specific activity and concentration as required) education. The reaction was initiated by addition of Mg / ATP mixture. After 40 minutes of incubation at room temperature, the reaction was stopped by adding phosphoric acid to a concentration of 0.5%. 10 [mu]L of the reaction was then spotted onto a P30 filter pad, washed four times for 4 minutes in 0.425% phosphoric acid and once in methanol before drying and scintillation counting.
[0341] Compound A was tested against Flt-3 using the Eurofins standard KinaseProfiler assay as described above. Compound A was also tested against IRAK1 and Flt-3(D835Y) using the same protocol as the substrates myelin basic protein (MBP) and ...
Embodiment 2
[0352] Example 2: In vitro assay of AML model MV4-11
[0353] The CellTiter Glo Luminescent Cell Viability Assay is a highly sensitive homogeneous assay for the determination of the number of viable cells in culture based on the quantification of ATP levels in metabolically active cells. Addition of CTG reagent results in cell lysis and a luminescent signal proportional to the amount of ATP present. The amount of ATP is directly proportional to the number of cells present. Luminescence was measured using a multilabel reader capable of measuring luminescence. An increase or decrease in cell number causes a corresponding change in luminescence levels, indicating the effect of the test material on cell proliferation.
[0354] Preparation of solutions / reagents
[0355] Preparation of CTG reagent:
[0356] Thaw CellTiter-Glo Buffer and equilibrate to room temperature. The lyophilized CellTiter-Glo substrate was brought to room temperature. CTG reagent was prepared by mixing...
Embodiment 3
[0377] Example 3: Inhibition of Cell Proliferation in the MV4-11 Xenograft Model
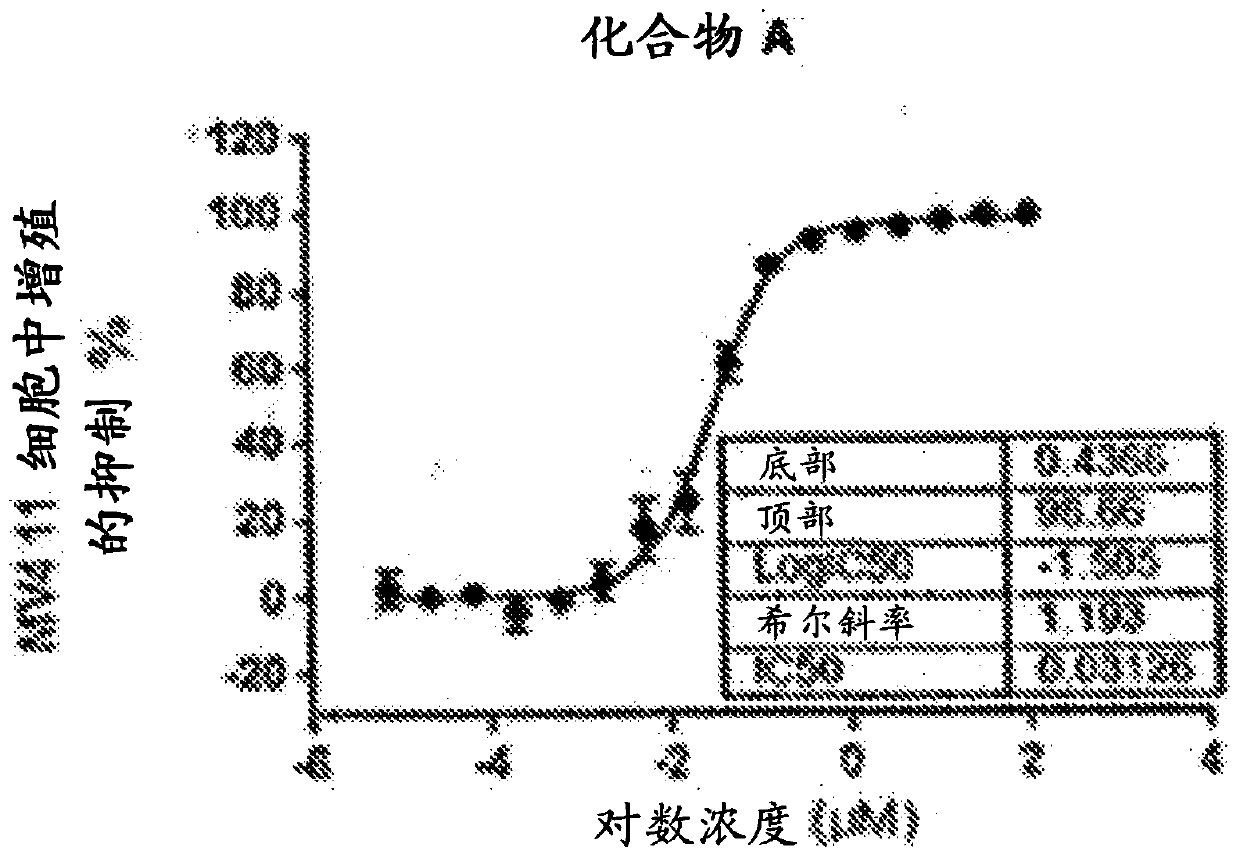
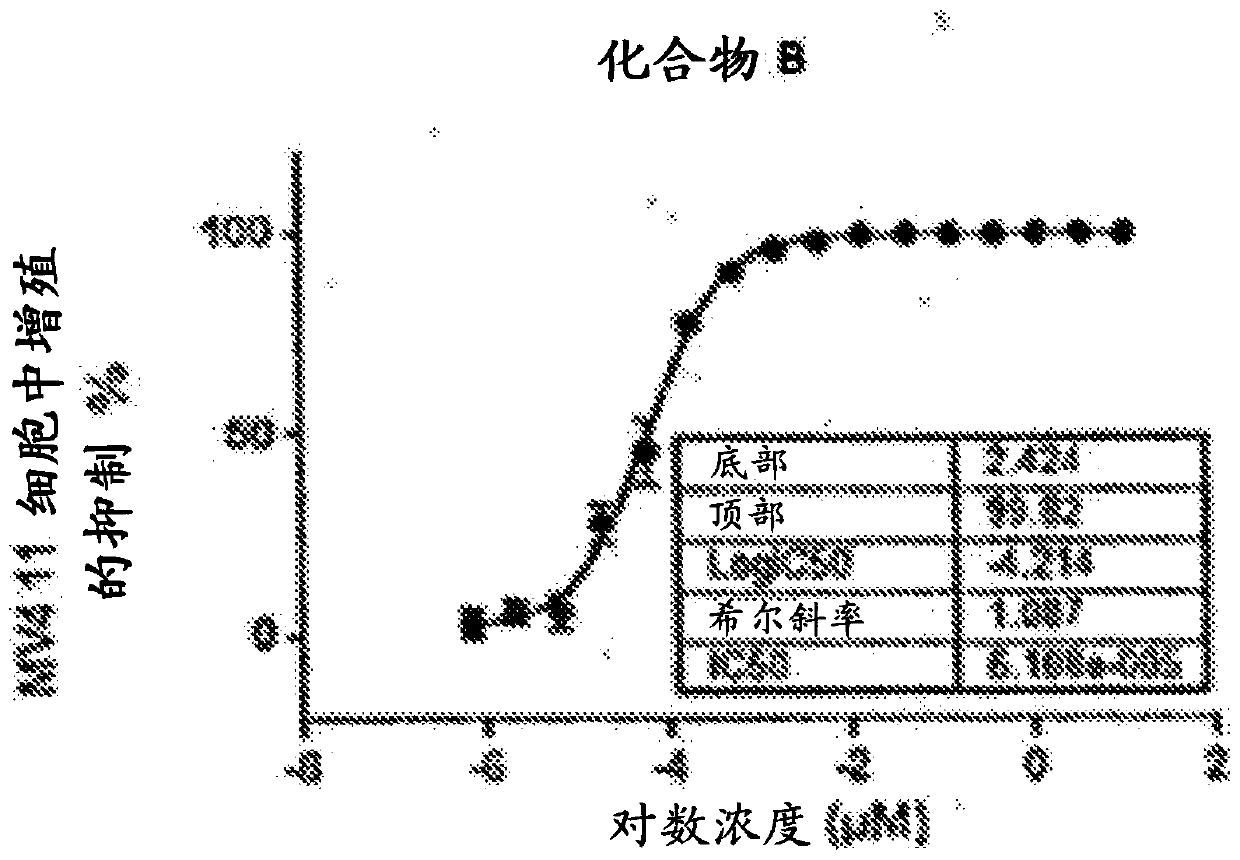
[0378] Using the procedure of Example 2, Compounds A and B were evaluated to determine % inhibition of proliferation in MV4-11 cells. IC of Compound A 50 0.031μM ( Figure 2A ), IC of Compound B 50 6.1e-005μM ( Figure 2B ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com