Preparation method of lipid-lowering medicinal tablet with good sustained-release effect
A tablet and lipid-lowering technology, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, and pharmaceutical formulations, etc., can solve the problem of unstable drug release rate, unstable blood drug concentration of patients, and difficulty in screening excipients. and other problems, to achieve the effect of great market competitiveness, promotion and use value, significant control effect, and good slow-release effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
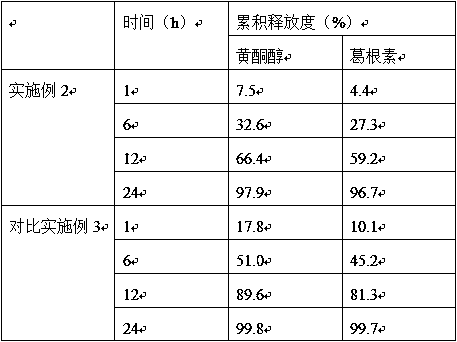
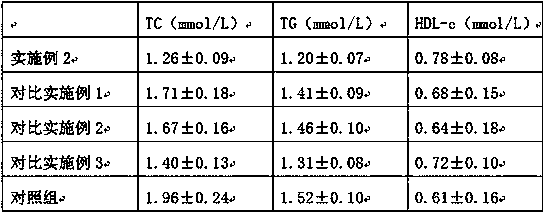
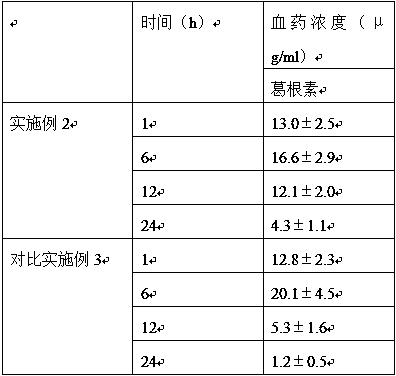
Examples
Embodiment 1
[0037] A preparation method for a lipid-lowering tablet with good sustained-release effect, comprising the steps of:
[0038] (1) The raw materials of traditional Chinese medicine are weighed for later use:
[0039] Weigh the following traditional Chinese medicine raw materials according to the corresponding parts for later use: 20 parts of Panax notoginseng, 5 parts of Hawthorn, 3 parts of Polygonum multiflorum, 2 parts of Salvia miltiorrhiza, 4 parts of Pueraria lobata;
[0040] (2) Cleaning, drying and crushing treatment:
[0041] Clean the Panax notoginseng, hawthorn, Polygonum multiflorum, Salvia and Pueraria root weighed in step (1) with clean water, and then dry them, and finally put the Panax notoginseng, hawthorn, Polygonum multiflorum, Salvia and Puerariae together into the grinder for crushing After processing, take it out and mix it with Chinese medicine powder for later use;
[0042] (3) Steam explosion treatment:
[0043] Put the mixed traditional Chinese medi...
Embodiment 2
[0064] A preparation method for a lipid-lowering tablet with good sustained-release effect, comprising the steps of:
[0065] (1) The raw materials of traditional Chinese medicine are weighed for later use:
[0066] Weigh the following traditional Chinese medicine raw materials according to the corresponding parts for later use: 23 parts of Panax notoginseng, 7 parts of Hawthorn, 5 parts of Polygonum multiflorum, 4 parts of Salvia miltiorrhiza, 6 parts of Pueraria lobata;
[0067] (2) Cleaning, drying and crushing treatment:
[0068] Clean the Panax notoginseng, hawthorn, Polygonum multiflorum, Salvia and Pueraria root weighed in step (1) with clean water, and then dry them, and finally put the Panax notoginseng, hawthorn, Polygonum multiflorum, Salvia and Puerariae together into the grinder for crushing After processing, take it out and mix it with Chinese medicine powder for later use;
[0069] (3) Steam explosion treatment:
[0070] Put the mixed traditional Chinese medi...
Embodiment 3
[0091] A preparation method for a lipid-lowering tablet with good sustained-release effect, comprising the steps of:
[0092] (1) The raw materials of traditional Chinese medicine are weighed for later use:
[0093] Weigh the following traditional Chinese medicine raw materials according to the corresponding parts for later use: 25 parts of Panax notoginseng, 8 parts of Hawthorn, 6 parts of Polygonum multiflorum, 5 parts of Salvia miltiorrhiza, 7 parts of Pueraria lobata;
[0094] (2) Cleaning, drying and crushing treatment:
[0095]Clean the Panax notoginseng, hawthorn, Polygonum multiflorum, Salvia and Pueraria root weighed in step (1) with clean water, and then dry them, and finally put the Panax notoginseng, hawthorn, Polygonum multiflorum, Salvia and Puerariae together into the grinder for crushing After processing, take it out and mix it with Chinese medicine powder for later use;
[0096] (3) Steam explosion treatment:
[0097] Put the mixed traditional Chinese medic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com