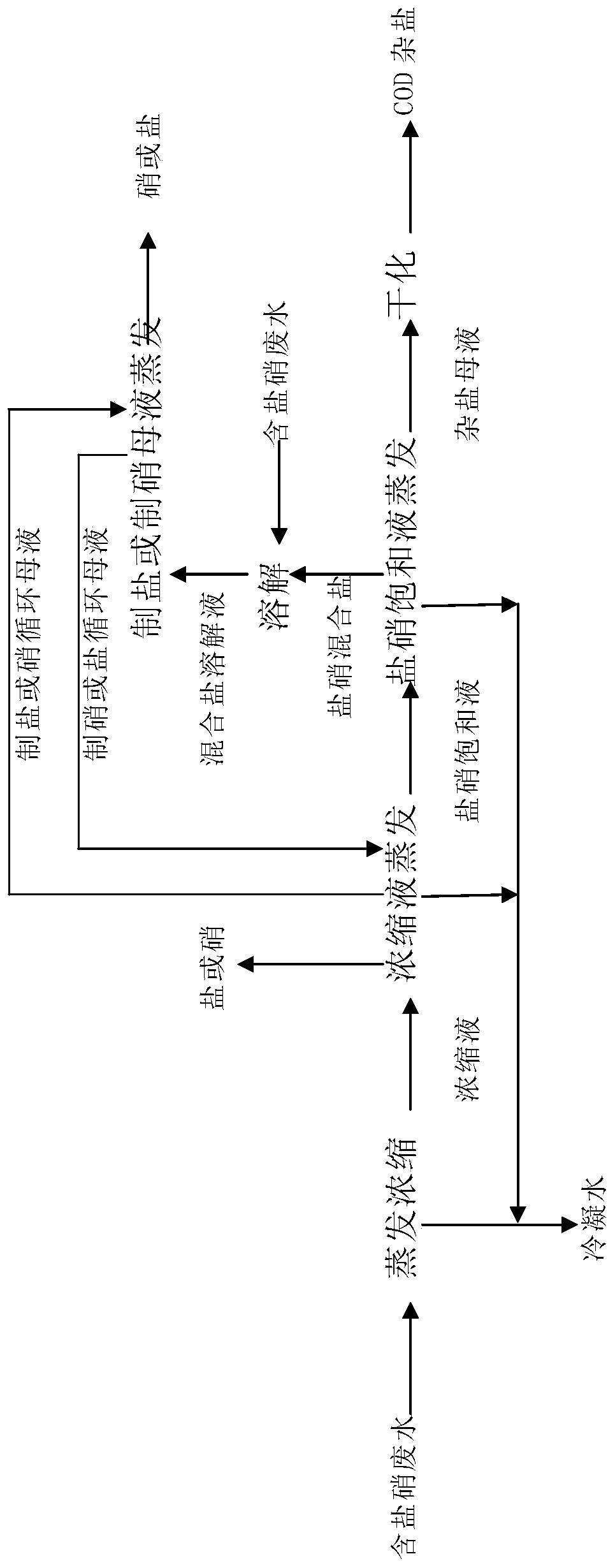
Process for separating nitrates, COD salts and sulfates by evaporating wastewater containing nitrates, COD salts and sulfates
A technology of nitrate and salt nitrate, which is applied in the field of evaporation and separation of nitrate COD salt nitrate wastewater containing nitrate COD salt nitrate, can solve the complex process flow of the wastewater pretreatment system, the large consumption of refrigeration equipment and cold energy, and the energy consumption of refrigeration Dashi water Glauber's salt and other problems, to achieve the effect of strong adaptability of raw materials, low TOC, high yield of salt salt
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 (nitrate type brine): get 200m 3 Salt salt wastewater (NaCl 5.29g / l, NaCl 2 SO 4 39.91g / l, CaSO 4 0.007g / l, MgSO 4 0.006g / l, COD corresponding to reducing substances 0.058g / l, NaNO 3 0.20g / l) as raw material, the 200m 3 Salt salt wastewater is evaporated (evaporation temperature 100°C) and concentrated to obtain fresh water and 25.46m 3 Concentrate (NaCl 46.5g / l, Na 2 SO 4 313.5g / l, CaSO 4 0.055g / l, MgSO 4 0.047g / l, COD corresponding to reducing substances 0.458g / l, NaNO 3 1.579g / l); the 25.46m 3 Concentrate (NaCl 46.5g / l, Na 2 SO 4 313.5g / l, CaSO 4 0.055g / l, MgSO 4 0.047g / l, COD corresponding to reducing substances 0.458g / l, NaNO 3 1.579g / l) and 16.81m 3 Salt circulating mother liquor (NaCl290.5g / l, Na 2 SO 4 64.5g / l, CaSO 4 0.11g / l, MgSO 4 0.094g / l, COD corresponding to reducing substances 3.03g / l, NaNO 3 10.452g / l) mixed evaporation (evaporation temperature 100°C) to obtain 7.879 tons of nitrate, fresh water and 23.57m ...
Embodiment 2
[0035] Embodiment 2 (nitrate type brine): get 200m 3 Salt salt wastewater (NaCl 20.55g / l, NaCl 2 SO 4 20.80g / l, COD corresponding to reducing substances 0.206g / l) as raw materials, the 200m 3 Salt salt wastewater is evaporated (evaporation temperature 100°C) and concentrated to obtain fresh water and 22.91m 3 Concentrate (NaCl 179.40g / l, NaCl 2 SO 4 181.58g / l, COD corresponds to reducing substances 1.799g / l); the 22.91m 3 Concentrate (NaCl 179.40g / l, NaCl 2 SO 4 181.58g / l, COD corresponds to reducing substances 1.799g / l) and 56.67m 3 Salt circulating mother liquor (NaCl 290.5g / l, NaCl 2 SO 4 64.5g / l, COD corresponds to reducing substances 3.175g / l) mixed evaporation (evaporation temperature 100°C) to obtain 4.145 tons of nitrate, fresh water and 79.47m 3 Saturated salt solution (NaCl 308g / l, NaCl 2 SO 4 55g / l, COD corresponds to reducing substances 3.175g / l); the 79.47m 3 Saturated salt solution (NaCl 308g / l, NaCl 2 SO 4 55g / l, COD corresponds to 13.01m in ...
Embodiment 3
[0036] Embodiment 3 (salt salt type brine): get 200m 3 Salt salt wastewater (NaCl 39.91g / l, NaCl 2 SO 4 5.92g / l, CaSO 4 0.007g / l, MgSO 4 0.006g / l, COD corresponding to reducing substances 0.058g / l, NaNO 3 0.20g / l) as raw material, the 200m 3 Salt salt wastewater is evaporated (evaporation temperature 100°C) and concentrated to obtain fresh water and 25.46m 3 Concentrate (NaCl 313.5g / l, Na 2 SO 4 46.5g / l, CaSO 4 0.055g / l, MgSO 4 0.047g / l, COD corresponding to reducing substances 0.458g / l, NaNO 3 1.579g / l); the 25.46m 3 Concentrate (NaCl 313.5g / l, Na 2 SO 4 46.5g / l, CaSO 4 0.055g / l, MgSO 4 0.047g / l, COD corresponding to reducing substances 0.458g / l, NaNO 3 1.579g / l) and 93.78m 3 Nitrate circulating mother liquor (NaCl305g / l, Na 2 SO 4 55g / l, CaSO 4 0.11g / l, MgSO 4 0.094g / l, COD corresponding to reducing substances 3.03g / l, NaNO 3 10.452g / l) mixed evaporation (evaporation temperature 55°C) to obtain 7.815 tons of salt, fresh water and 119.99m 3 S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com