Recovery process for wastewater with high salt COD (chemical oxygen demand)
A waste water recycling and process technology, applied in the field of high-salt COD waste water recycling process, can solve the problems of long process flow, high production cost, difficult utilization, etc., and achieve the effects of environmental protection and green process, strong adaptability of raw materials, and low production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
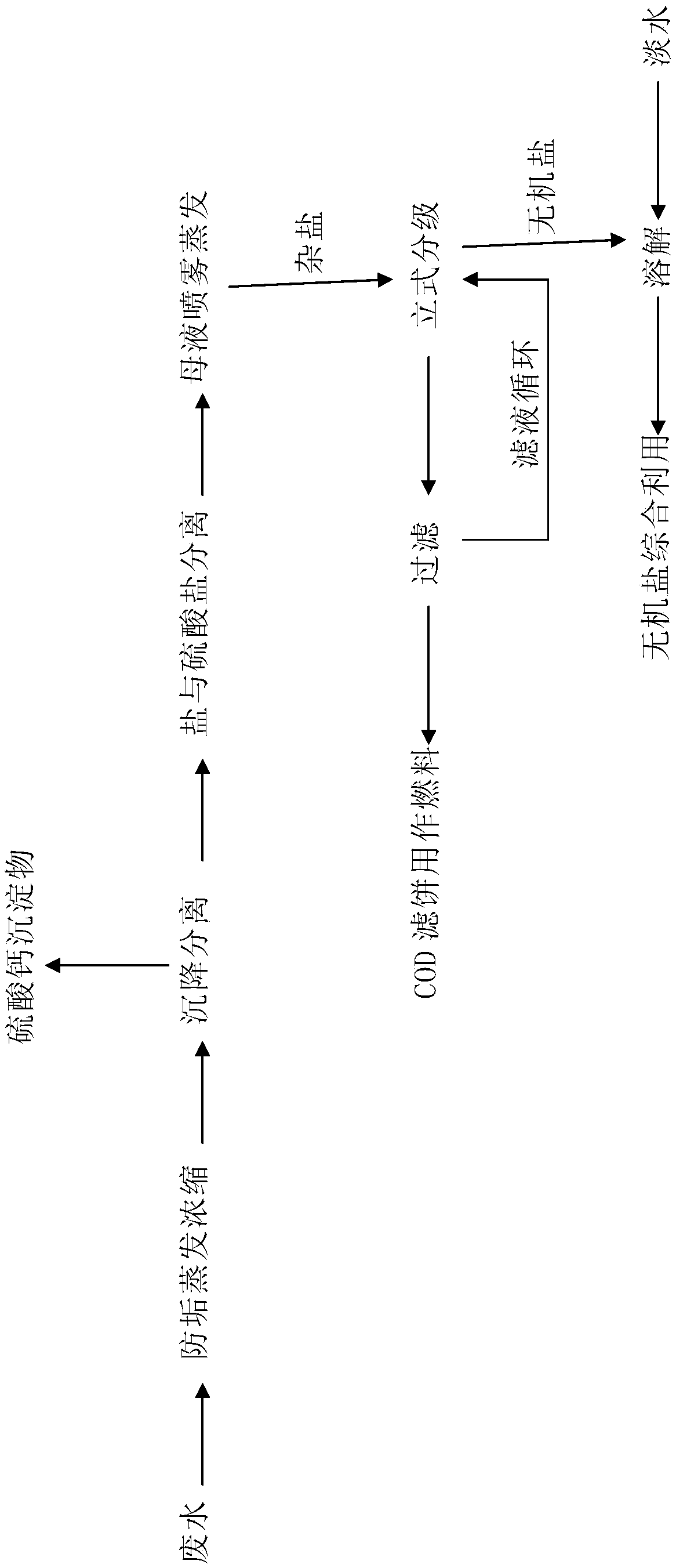
[0031] Embodiment 1 (cold process high magnesium calcium type brine):
[0032] Take 1000m 3 Wastewater (NaCl 3.4g / l, MgSO 4 0.1g / l, CaSO 4 0.05g / l, COD corresponding to reducing substances 0.03g / l, NaNO 3 0.03g / l) as raw material, the waste water is evaporated and concentrated under the condition of controlling its concentration with sand as an anti-scaling agent (evaporating temperature 40-140°C) to obtain 990 tons of fresh water and 10m 3 Nearly saturated solution (calcium sulfate supersaturated precipitate) (NaCl340g / l, MgSO 4 10g / l, CaSO 4 3.0g / l, COD corresponds to reducing substances 3g / l, NaNO 3 3g / l); 10m 3 Solid-liquid separation of nearly saturated solution to obtain 20kg calcium sulfate precipitate, 10m 3 Brine clarification solution (NaCl 340g / l, MgSO 4 10g / l, CaSO 4 3.0g / l, COD corresponds to reducing substances 3g / l, NaNO 3 3g / l); 10m 3 The brine clarified liquid was subjected to solid-liquid separation through evaporative crystallization (evap...
Embodiment 2
[0033] Embodiment 2 (cold process nitrate co-production):
[0034] Take 1000m 3 Wastewater (NaCl 3.4g / l, NaCl 2 SO 4 3.4g / l, MgSO 4 0.01g / l, CaSO 4 0.05g / l, COD corresponding to reducing substances 0.03g / l, NaNO 3 0.03g / l) as raw material, the waste water is evaporated and concentrated under the condition of controlling its concentration with sand as an anti-scaling agent (evaporating temperature 40-140°C) to obtain 980 tons of fresh water and 20m 3 Nearly saturated solution (calcium sulfate supersaturated precipitate) (NaCl 170g / l, NaCl 2 SO 4 170g / l, MgSO 4 0.50g / l, CaSO 4 1.0g / l, COD corresponding to reducing substances 1.5g / l, NaNO 3 1.5g / l); 20m 3 Solid-liquid separation of nearly saturated solution to obtain 30kg calcium sulfate precipitate, 20m 3 Brine clarification solution (NaCl 170g / l, NaCl 2 SO 4 170g / l, MgSO 4 0.50g / l, CaSO 4 1.0g / l, COD corresponding to reducing substances 1.5g / l, NaNO 3 1.5g / l); 20m 3 The brine clarified liquid was su...
Embodiment 3
[0035] Embodiment 3 (cold process salt nitrate co-production):
[0036] Take 1000m 3 Wastewater (NaCl 6.0g / l, Na 2 SO 4 0.80g / l, MgSO 4 0.01g / l, CaSO 4 0.05g / l, COD corresponding to reducing substances 0.03g / l, NaNO 3 0.03g / l) as raw material, the waste water is evaporated and concentrated under the condition of controlling its concentration with sand as an anti-scaling agent (evaporating temperature 40-140°C) to obtain 980 tons of fresh water and 20m 3 Nearly saturated solution (NaCl 300g / l, NaCl 2 SO 4 40g / l, MgSO 4 0.50g / l, CaSO 4 1.0g / l, COD corresponding to reducing substances 1.5g / l, NaNO 3 1.5g / l); 20m 3 Solid-liquid separation of nearly saturated solution to obtain 30kg calcium sulfate precipitate, 20m 3 Brine clarification solution (NaCl 300g / l, NaCl 2 SO 4 40g / l, MgSO 4 0.50g / l, CaSO 4 1.0g / l, COD corresponding to reducing substances 1.5g / l, NaNO 3 1.5g / l); 20m 3 The brine clarified liquid was subjected to solid-liquid separation through e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com