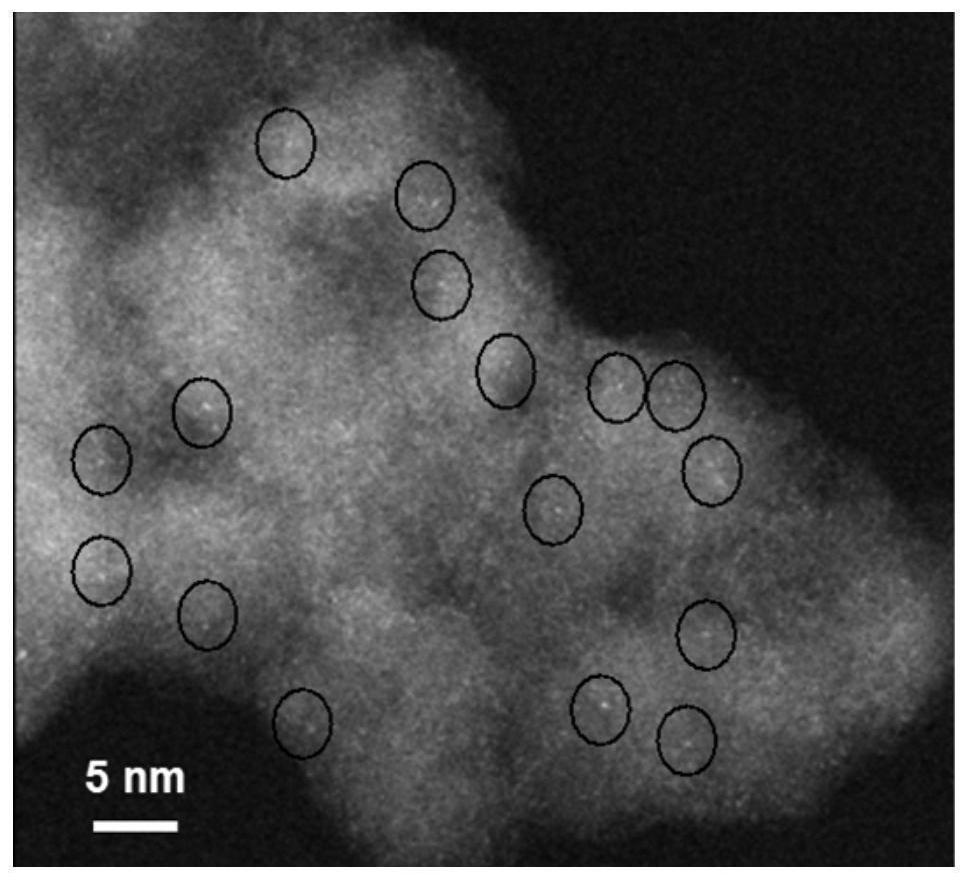
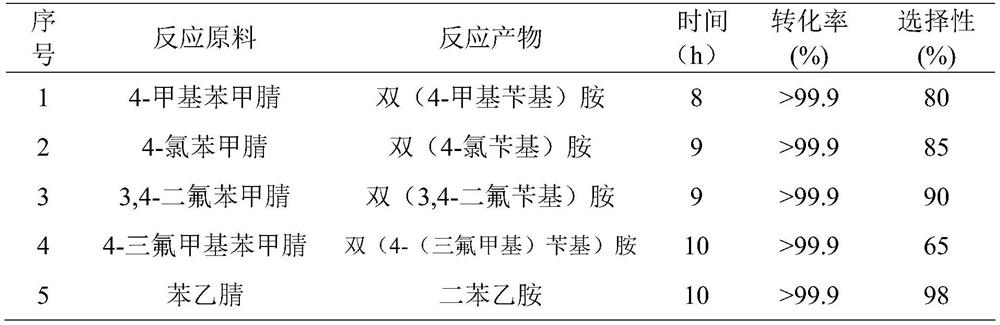
Application of nanocarbon-supported single-atom palladium-based catalysts in the catalytic hydrogenation of nitrile compounds to prepare secondary amines
A technology for catalytic hydrogenation and compounding, which is applied in the preparation of amino compounds, the preparation of organic compounds, catalysts for physical/chemical processes, etc., can solve problems such as low selectivity, and achieve the effects of good repeatability, mature production technology and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A single-atom palladium-based catalyst (Pd 0.1 / ND@G) 20mg into a 50ml pressure-resistant reaction bottle, add 4mmol of ammonia borane, then add 10ml of methanol solution containing 0.5mmol of the reaction substrate, and react at a reaction temperature of 60°C for 8h. After the reaction, the conversion rate of benzonitrile is 55%, the selectivity of the product dibenzylamine is 98%, and the total selectivity of other by-products is 2%.
Embodiment 2
[0028] A single-atom palladium-based catalyst (Pd 0.1 / ND@G) 30mg was added to a 50ml pressure-resistant reaction bottle, 4mmol of ammonia borane was added, and 10ml of methanol solution containing 0.5mmol of the reaction substrate was added, and the reaction was carried out at a reaction temperature of 60°C for 8h. After the reaction, the conversion rate of benzonitrile is >99.9%, the selectivity of the product dibenzylamine is 98%, and the total selectivity of other by-products is 2%.
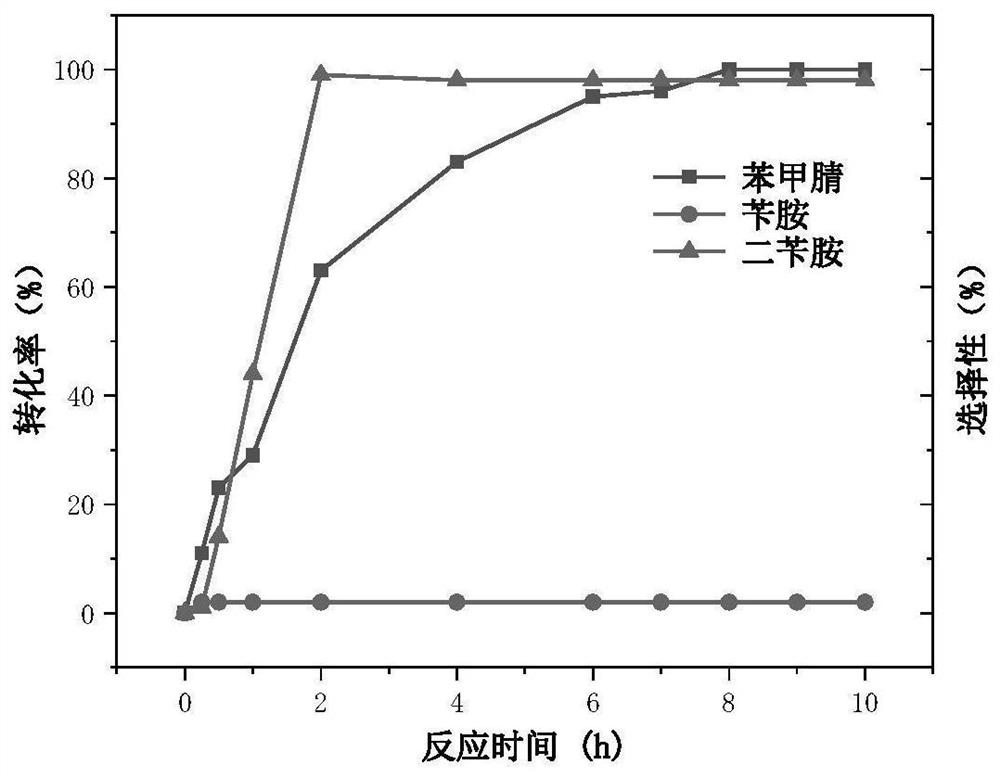
[0029] Under the condition that other conditions remain unchanged, only changing the reaction time, the activity and selectivity of the catalyst at different reaction times will change as follows: figure 2 shown.
Embodiment 3
[0033] A single-atom palladium-based catalyst (Pd 0.1 / ND@G) 30mg was added to a 50ml pressure-resistant reaction bottle, 3mmol of ammonia borane was added, and 10ml of methanol containing 0.5mmol of the reaction substrate was added, and the reaction was carried out at a reaction temperature of 60°C for 8h. After the reaction, the conversion rate of benzonitrile was 54%, the selectivity of the product dibenzylamine was 98%, and the total selectivity of other by-products was 2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com