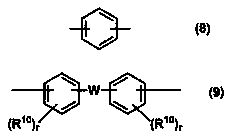
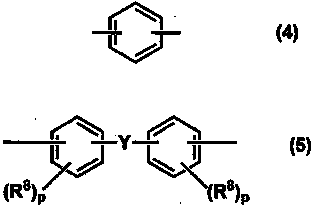
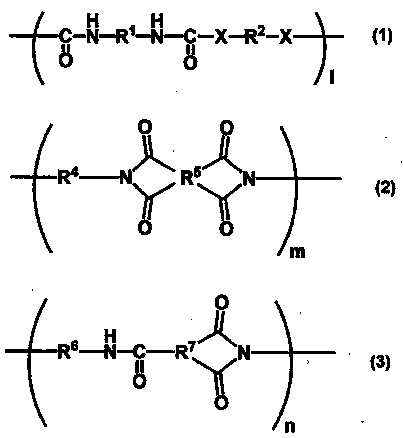
Resin, resin composition, nonwoven fabric using same, fiber product, separator, secondary battery, and method for producing electric double layer capacitor and nonwoven fabric
A technology of resin composition and non-woven fabric, applied in the direction of single-component synthetic polymer rayon, dry spinning method, fiber treatment, etc., can solve the problems of separator shrinkage, melting, electrode short circuit, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0101] In order to demonstrate this invention in more detail, although an Example is used and demonstrated more concretely, this invention is not interpreted as being limited by these Examples. In addition, evaluation of resin etc. was performed by the following method.
[0102] (1) Production of non-woven fabrics
[0103]The resin solution was sprayed at 40 μL / min with an electrospinning device (manufactured by Kato-Tec Co., Ltd., NEU nanofiber electrospinning unit), and the resin solution was sprayed on an aluminum foil with a mass per unit area of 5 g / m 2 Form a nonwoven fabric. The nozzle used an 18G (0.94 mm inner diameter) unbeveled needle with a distance of 15 cm to the collection device. When setting the voltage, the front part of the nozzle was visually confirmed for each sample, and the resin solution was stably maintained in a conical (Taylor cone) shape at the front part of the nozzle. The obtained nonwoven fabric on the aluminum foil was vacuum-dried at 150° ...
Synthetic example 1
[0115] Synthesis Example 1: Synthesis of Resin A-1
[0116] In a dry nitrogen stream, N,N-dimethylacetamide (manufactured by Fujifilm Wako Pure Chemical Industries, Ltd., product name "N,N-dimethylacetamide (super-dehydrated), hereinafter referred to as DMAc" Dissolve 10.27 g (0.095 mol) of p-phenylenediamine (manufactured by Fuji Film Wako Pure Chemical Industries, Ltd., hereinafter referred to as PDA) in 82.13 g. Add isophorone diisocyanate (Tokyo Chemical Industry ( Co., Ltd., product name "Isophorone Diisocyanate (mixture of isomers)", hereinafter referred to as IPDI) 22.23g (0.10mol), the flask was immersed in an oil bath at 60°C and stirred for 4 hours. Then, hard 2.70 g (0.01 mol) of tallow amine (manufactured by Tokyo Chemical Industry Co., Ltd., product name "Stearylamine") was stirred in an oil bath at 60° C. for 2 hours to obtain a 30% by mass DMAc solution of resin A-1. Resin A- 1 Containing 50 mol% or more (substantially 100 mol%) of the structure represented by ...
Synthetic example 2
[0117] Synthesis example 2: the synthesis of resin A-2
[0118] In a dry nitrogen stream, in a flask, 13.19 g of DMAc was dissolved in 13.19 g of 3,3'-diaminodiphenylsulfone (manufactured by Tokyo Chemical Industry Co., Ltd., product name "Bis(3-aminophenyl) Sulfone", hereinafter referred to as 3,3'-DDS) 23.59 g (0.095 mol). After adding 22.23 g (0.10 mol) of IPDI little by little thereto, the flask was immersed in a 60° C. oil bath and stirred for 4 hours. Then, 2.70 g (0.01 mol) of stearylamine was put into the flask, and it stirred in the oil bath of 60 degreeC for 2 hours, and obtained the 30 mass % DMAc solution of resin A-2. Resin A-2 contains more than 50 mol% (substantially 100 mol%) of the structure represented by the general formula (1) in the entire structure, and the end of the main chain has an alkyl group with 18 carbon atoms, and X in the general formula (1) is The structure represented by -NH-, R 1 is a cyclic aliphatic hydrocarbon group, R 2 It corresponds...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com