Method for preparing chromium chloride hexahydrate by using sodium chromate
A technology of chromium chloride hexahydrate and sodium chromate, applied in chromium halide, alkali metal chloride, chemical industry and other directions, can solve the problems of large consumption of hazardous waste and reducing substances, large consumption of energy consumption, etc. The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A method utilizing sodium chromate to prepare chromium chloride hexahydrate, comprising the following steps:
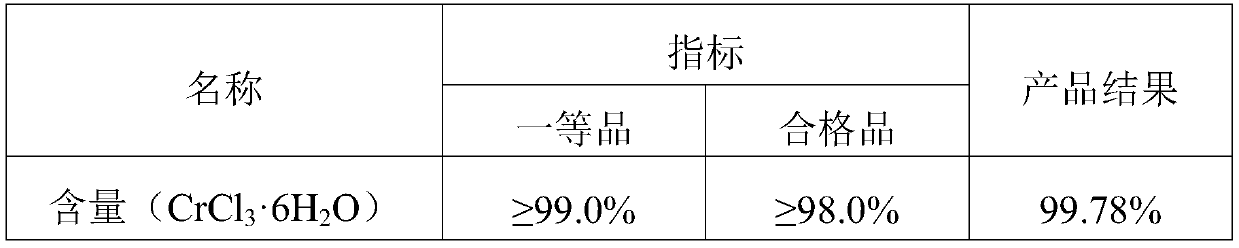
[0028] Add 600g sodium chromate to 556g HCl, heat to boiling, react for ten minutes, filter to remove sodium chloride, pour the filtrate into the reaction tank, add a condensing device, and pump 1017.76g HCl and 69.20g ethanol into In the tank, add the reaction time for a total of 4 hours, the reaction temperature is 85°C, filter out the undissolved sodium chloride, evaporate and concentrate at 100°C until the Baume degree is 49 Baume degrees, filter the sodium chloride once, and then add some water to 47 Baume degrees, cooled to 35 degrees Celsius, crystallized for 4 hours to precipitate chromium chloride hexahydrate, and then centrifuged in a centrifuge for half an hour to obtain chromium chloride hexahydrate crystals, and the prepared chromium chloride hexahydrate crystals were detected. The index parameters are shown in Table 1;
[0029] Table 1
[0030] ...
Embodiment 2
[0033] A method utilizing sodium chromate to prepare chromium chloride hexahydrate, comprising the following steps:
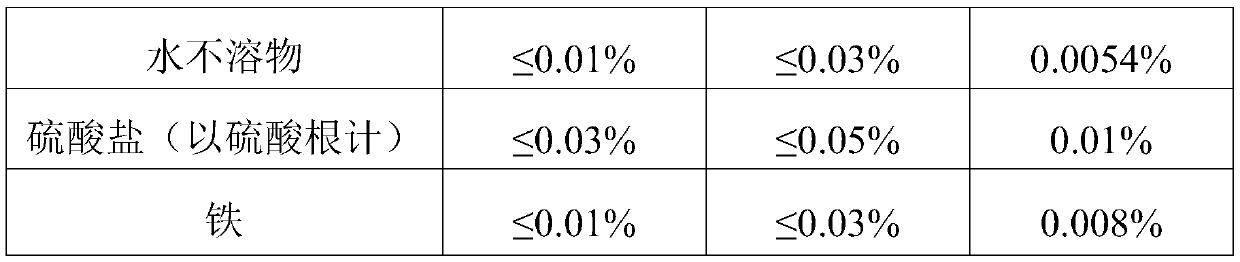
[0034] Add 600g sodium chromate to 559g HCl, heat to boiling, react for ten minutes, filter to remove sodium chloride, pour the filtrate into the reaction tank, add a condensing device, and pump 881.7g HCl and 69.26g ethanol into In the tank, add the reaction time for a total of 4 hours, the reaction temperature is 85°C, filter out the undissolved sodium chloride, evaporate and concentrate at 100°C until the Baume degree is 49 Baume degrees, filter the sodium chloride once, and then add some water to 47 Baume degrees, cooled to 35 degrees Celsius, crystallized for 4 hours to precipitate chromium chloride hexahydrate, and then centrifuged in a centrifuge for half an hour to obtain chromium chloride hexahydrate crystals, and the prepared chromium chloride hexahydrate crystals were detected. The index parameters are shown in Table 2;
[0035] Table 2
[0036] ...
Embodiment 3
[0038] A method utilizing sodium chromate to prepare chromium chloride hexahydrate, comprising the following steps:
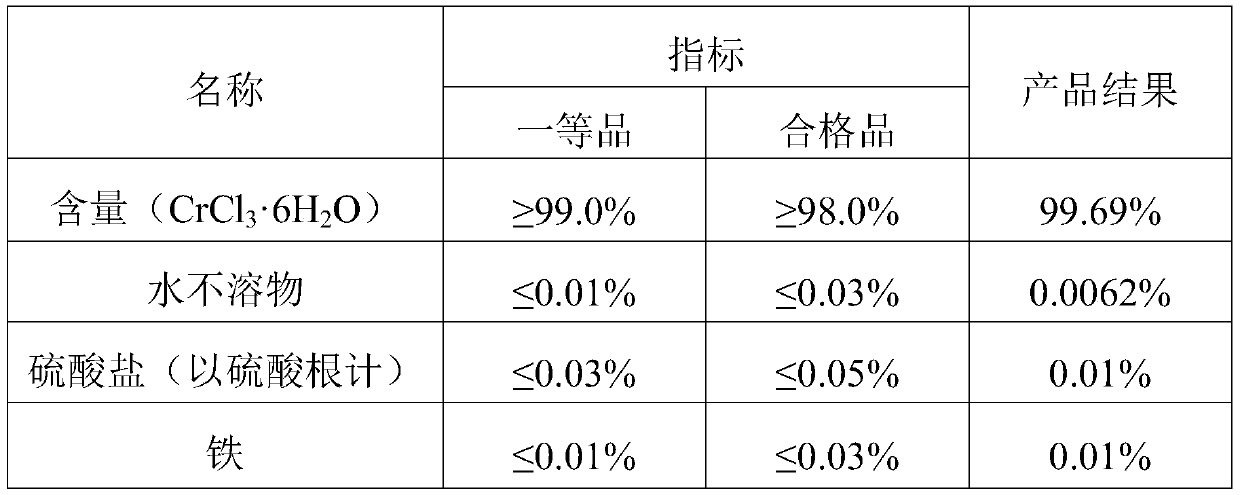
[0039] Add 600g sodium chromate to 558g HCl, heat to boiling, react for ten minutes, filter to remove sodium chloride, pour the filtrate into the reaction tank, add a condensing device, and pump 892.7g HCl and 40.22g ethanol into In the tank, add the reaction time for a total of 4 hours, the reaction temperature is 85°C, filter out the undissolved sodium chloride, evaporate and concentrate at 100°C until the Baume degree is 49 Baume degrees, filter the sodium chloride once, and then add some water to 47 Baume degrees, cooled to 35 degrees Celsius, crystallized for 4 hours to precipitate chromium chloride hexahydrate, and then centrifuged in a centrifuge for half an hour to obtain chromium chloride hexahydrate crystals, and the prepared chromium chloride hexahydrate crystals were detected. The index parameters are shown in Table 3;
[0040] table 3
[0041] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com